Research - (2025) Volume 20, Issue 2
Synthesis, characterization, and evaluation of transition metal complexes of 4-Amino-5-Phenyl-4H-1,2,4-Triazole 3-Thiol as anti-breast cancer
Israa A. Bachay1* and Noor H. Naser2Received: 21-Apr-2025, Manuscript No. gpmp-25-166087; Editor assigned: 23-Apr-2025, Pre QC No. P-166087; Reviewed: 05-May-2025, QC No. Q-166087; Revised: 23-Jun-2025, Manuscript No. R-166087; Published: 30-Jun-2025
Abstract
Background: Breast cancer is a disease where cells in the breast grow out of control, forming lumps. If these lumps are not treated, they can spread to other parts of the body. The disease often starts in the milk ducts or lobules. Early forms, called 'in situ', can be found and treated before they become life-threatening. Aim: evaluation the novel synthesize a series of 1,2,4-triazole-based metal complexes and evaluate their structural features and cytotoxic activity against breast cancer cells. Methodology: A multi-step synthetic route was employed starting from ethyl benzoate to obtain the triazole ligand (Compound III), which was subsequently complexed with various metal ions (Ni²⁺, Co²⁺, Cd²⁺, Cr³⁺, Zn²⁺) to afford complexes IVa–IVe. The compounds were characterized using FT-IR and ¹H NMR spectroscopy. Cytotoxicity was assessed in vitro against MCF-7 (cancerous) and MCF-10a (non-tumorigenic) breast cell lines using MTT assay, with IC₅₀ values determined from triplicate measurements. Results: Spectroscopic analysis confirmed the successful formation of the triazole core and its coordination with metal ions through nitrogen and sulfur atoms. Among the tested complexes, IVc (Cd²⁺) and IVe (Zn²⁺) exhibited the highest cytotoxicity against MCF-7 cells (IC₅₀ = 28.45 ± 2.34 μM and 52.57 ± 4.72 μM, respectively) with significantly reduced toxicity toward MCF-10a cells, indicating selective anticancer activity. Conclusion: The results highlight the importance of metal coordination in enhancing the anticancer potential of triazole derivatives. Complexes IVc and IVe, in particular, show promise as lead compounds for further development in anticancer drug discovery.
Keywords
Cancer; Heterocyclic; Triazole; Metal complexes
Introduction
The latest statistics from the World Health Organization indicate that cancer is the second biggest cause of death globally. Annually, around 9.6 million fatalities occur, or almost one-fourth of total mortality. Numerous anticancer pharmaceuticals have undergone substantial enhancements in recent years. Nevertheless, most presently accessible anti-cancer pharmaceuticals are ineffective, and adverse consequences such as drug-induced impairment may occur. Consequently, developing and enhancing new, safe, and effective long-term cancer therapies with reduced side effects is imperative [1]. Heterocyclic chemistry has evolved into a distinct discipline within chemistry, characterized by a rich history, contemporary relevance, and promising future possibilities. Nitrogen, oxygen, and sulfur are regarded as the most prevalent heteroatoms. Heterocyclic substances are regarded as a significant category of organic chemicals because of their significance in pharmaceuticals and industrial research [2]. Triazoles are five-membered heterocyclic molecules having the chemical formulae C2H3N3, bearing three atoms of nitrogen and two atoms of carbon [3]. There are two categories of triazoles, which: 1,2,3-triazoles and 1,2,4-triazoles [4,5]. Both of these two types of triazole, which are essential constituents of various therapeutic medicines, and their analogues have consistently captivated medicinal chemists [6]. Triazole-containing heterocycles are lead molecule frameworks that have garnered researchers' attention due to their extensive array of biological activities, including anticancer, antimicrobial [7], antitubercular [8], anti-HIV [9], anticonvulsant [10], antibacterial [11], anti-inflammatory [12], analgesic, and antiviral properties [13]. Triazoles are regarded as effective coordinating ligands due to their incorporation of each hard nitrogen and soft sulfur atom in the thioamide group [14]. This ligand possesses donor groups that coordinate with a broad spectrum of metal ions [15]. The tautomeric form might appear in triazole [16]. The possible coordinating sites include: (I) the sulfur of the thiol group, (II) the nitrogen of the main amino group, and (III) the two nitrogen atoms at positions 1 and 2 in the triazole ring system [17]. This ligand has an S=C_N_N unit that facilitates bidentate coordination to metal ions via amine and thiol substitutions, resulting in the formation of a stable five-membered ring [18]. Consequently, this ligand is polydentate, and it has been demonstrated and empirically validated that complex formed with polydentate ligands are referred to as chelate complexes. They are generally more stable than complexes formed from monodentate ligands. Moreover, five or six-membered chelates are the most prevalent and stable [19]. A novel thio-triazole compound with specific metals was synthesized [20]. This study details the production, characterization, and assessment of Ni (II), Co (II), Cd (II), Cr (II), and Zn (II) complexes with 4-amino-5-(phenyl)-4H-1,2,4-triazole-3-thiol.
Methodology
All reagents, starting ingredients, and solvents were acquired commercially and utilized without additional purification. The chemicals and anhydrous solvents employed in this study were obtained from various suppliers, including Merck, Reidel Dehaan, Sigma-Aldrich, and Thomas Baker. The Thomas-Hoover apparatus was utilized to determine melting points by the capillary tube technique. The College of Pharmacy at the University of Kufa employed a Shimadzu spectrophotometer to perform FT-IR scans and analyze spectra using potassium bromide (KBr) discs. Mashhad University of Medical Sciences utilized Bruker apparatus to obtain proton nuclear magnetic resonance (1H-NMR) data, with DSMO as solvent and tetramethylsilane, TMS, as the internal standard.
Chemical synthesis
The chemical protocol for the synthesis of triazole-metal complexes is prescribed in Fig. 1.

Fig. 1. Synthesis of triazole-metal complexes and their intermediates.
Synthesis of benzo hydrazide (compound I): The preparation of benzo hydrazide (I) was made by reacting Ethyl benzoate with hydrazine monohydrate according to Singh et al [21]. A combination of ethyl benzoate (14.3 ml, 100 mmol) and 99% hydrazine hydrate (14.6 ml, 300 mmol) was subjected to reflux for 15 minutes, after which sufficient 100% ethanol (10 ml) was introduced via the top of the condenser to achieve a clear solution. The mixture underwent reflux for two hours. Subsequently, evaporate the alcohol and refrigerate the remaining residue. The hydrazide crystals were isolated using filtering and recrystallized from ethanol to get white crystals. Physical data and including melting point and %yield, are provided in Tab. 1.
| Compound | Formula | Molecular weight | Melting point | Appearance | % Yield |
|---|---|---|---|---|---|
| Ethyl benzoate | C9H10O2 | 150.18 | -34°C | Colourless liquid | -------- |
| I | C7H8N2O | 136.15 | 106-108 | White crystals | 79% |
| II | C8H7KN2OS2 | 250.38 | 306-308 | White powder | 89% |
| III | C8H8N4S | 192.24 | 212-214°C | White crystals | 64% |
| IVa | C16H16N8NiS2 | 443.17 | 236-238 | Pale green crystals | 91% |
| IVb | C16H16CoN8S2 | 443.41 | 195-197 | Pale pink | 87% |
| IVc | C16H16CdN8S2 | 496.89 | 188-190 | White crystals | 79% |
| IVd | C16H16CrN8S2 | 436.48 | 200-202 | Pale | 81% |
| IVe | C16H16N8S2Zn | 449.86 | 180-182 | White powder | 76% |
Tab. 1. The characterization and physical data of the synthesized compound. Compound (I): Bezohydrazide. Compound (II): potassium 2-benzoyl hydrazine carbodithioate. Compound (III): 4-amino-5-phenyl 4-H-1,2,4-triazole-3-thiol. Compound (IVa): triazole–Nickel complex. (IVb): triazole-Cobalt complex. Compound (IVc): triazole-Cadmium complex. Compound (IVd): triazole-Chromium complex. Compound (IVe): triazole-Zinc complex.
Synthesis of potassium 2-benzoyl hydrazine carbodithioate (compound II): The compound was synthesized through the reaction of benzo hydrazide with carbon disulfide, as reported by Sarafroz et al. [22] “Benzo hydrazide (I) (13.6 g, 100 mmol) was introduced into a chilled solution of potassium hydroxide (8.4 g, 150 mmol) in 100% ethanol (250 ml) while stirring. Subsequently, carbon disulfide (11.5 ml, 190 mmol) was introduced dropwise, and the reaction mixture was agitated continuously for 12 hours at ambient temperature. The precipitated potassium dithiocarbazinate derivative was obtained through filtering, subsequently washed with anhydrous ether, and dried. The resultant potassium salt was utilized in the subsequent stage without additional purification”. Physical data and including melting point, and %yield is provided in Tab. 1.
Synthesis of 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol, compound (III): This compound is synthesized through the reaction of potassium dithiocarbazinate with 99% hydrazine hydrate, as reported by Singh et al [21]. A suspension of potassium dithiocarbazinate (II) (5 g, 20 mmol), hydrazine hydrate (99%) (2 ml, 40 mmol), and distilled water (20 ml) was refluxed for 3 hours. The reaction mixture turned green, hydrogen sulphide was released, and a homogeneous solution was formed. A white material was precipitated by diluting with 100 ml of cold water and acidifying with a few drops of 35% HCl. The result was filtered, rinsed with cold water (2×30 ml), and crystallized from ethanol to yield a white powder. Physical data and including melting point and %yield, are provided in Tab. 1.
Synthesisoftriazole-metalcomplexes(IVa-IVb): An ethanolic solution of appropriate metal salts [Nickel (II) chloride, Cobalt (II) chloride, Cadmium(II) chloride, Chromium (II) chloride, and Zinc (II) chloride] was combined with an ethanolic solution of 4-amino-5-(phenyl)-4H-1,2,4-triazole-3-thiol in a 1:2 (metal: ligand) molar ratio and refluxed for two hours, resulting in the formation of crystalline colored precipitates at room temperature. The resultant solids were washed with hot ethanoland subsequently dried and recrystallized from ethanol according to Majeed et al. [20]. Physical data and including melting point and %yield, are provided in Tab. 1.
In vitro cytotoxicity assessment
We assessed the anticancer efficacy of the newly synthesized triazole-metal complexes (compounds IVa, IVb, IVc, IVd, and IVe) by cell line tests utilizing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT). This study employed two cell types: MCF-7 is a breast cancer cell line used to study the effects of chemicals on malignant cells, and MCF10A is a normal breast epithelial cell line used to test the effects on non-cancerous cells. These cell lines were obtained from the Iranian National Cell Bank at the Pasteur Institute. MCF-7 cells were cultured in RPMI 1640 medium., whereas MCF10A cells were cultivated in DMEM: F12 medium. Both mediums were augmented with 10% fetal bovine serum (FBS), subsequently incorporating antibiotics (100 µg/ml streptomycin and 100 units/ml penicillin). The cells were preserved in a humidified environment at 37°C with 5% carbon dioxide. A trypsin/EDTA solution (Gibco) and phosphate-buffered saline (PBS) were employed for cell passaging. Cell viability was evaluated utilizing MTT from Sigma-Aldrich. Each cell type (MCF-7 and MCF10A) was individually cultivated in 96-well plates (1.4 × 10^4 cells per well with 200 µl of fresh media) following 24 hours (at 37°C in a 5% CO2 atmosphere). The supernatant was discarded, and 200 µl of MTT solution was added to each well, followed by four-hour incubation at 37°C. Following the conclusion of the incubation period, 100 µl of dimethyl sulfoxide (DMSO) was introduced to dissolve the formazan crystals, and the plate was placed on a shaker at 37°C until full dissolution of the crystals was verified. An ELISA reader (Model Wave xs2, BioTek) was employed to assess cell viability through absorbance at 570 nm and to compute the IC50 values.
Statistical analysis
The statistical analysis system -SAS (2018) program was used to detect the effect of different factors on study parameters. A t-test was used to significantly compare between means in the study [23].
Results
The physical analytical data, melting point, molecular weight, and percentage of the yield of the synthesized compounds are tabulated in Tab. 1.
Infra-red spectroscopy
Compound (I) FTIR (cm-1): 3414,3300 stretching of (NH2) respectively, 3224 stretching vibrations for (NH). 3022 (C-H) stretching vibration of aromatic. 1660 C=O stretching band of amide. 1618 (N-H) bending vibration of NH2. 1573 and 1447 C=C stretching vibration of aromatic. 1348 C-N stretching vibration. 1120 C-H (in plan bending) of aromatic. 883 C-H (out-of-plane bending) of aromatic. 684 N-H wagging vibration of NH2 [24].
Compound (II) FTIR (cm-1): 3327,3157 stretching vibration of (NH) of secondary amide and amine, respectively. 3062 (C-H) stretching vibration of aromatic. 1641 C=O stretching band of amide. 1575 (N-H) bending vibration of NH. 1539,1448 and 1398 C=C stretching vibration of aromatic. 1282 and 1240 C-H (in plan bending) of aromatic. 885 and 702 C-H (out-of-plane bending) of aromatic. 630 C-S stretching vibration.
Compound (III) FTIR (cm-1): 3298, 3145 stretching vibrations of (NH2) primary amine, 2995 (C-H) stretching vibration of aromatic.2762 S-H stretching vibration. 1640 (C=N) stretching vibration of triazole. 1485,1446 (C=C) stretching vibration of aromatic, 629 stretching vibrations of (C-S).
Compound (IVa) FTIR (cm-1): 3419, 3398 stretching vibrations of (NH2).3194,3115 stretching vibration (NH). 2941 (C-H) stretching vibration of aromatic.1629 (C=N) stretching vibration. 1494,1450, (C=C) stretching vibration of aromatic. 690 stretching vibration of C-S. 501 stretching vibration for M-N, and 443 for M-S.
Compound (IVb) FTIR (cm-1): 3425, 3298 stretching vibrations of (NH2).3194 stretching vibration (NH). 2997 (C-H) stretching vibration of aromatic.1635 (C=N) stretching vibration. 1494,1450, (C=C) stretching vibration of aromatic. 686 stretching vibration of C-S. 501 stretching vibration for M-N, and 459 for M-S.
Compound (IVc) FTIR (cm-1): 3471, 3425 stretching vibrations of (NH2).3199 stretching vibration (NH). 3080 (C-H) stretching vibration of aromatic.1624 (C=N) stretching vibration. 1489,1448, (C=C) stretching vibration of aromatic. 692 stretching vibration of C-S. 509 stretching vibration for M-N, and 441 for M-S.
Compound (IVd) FTIR (cm-1): 3298, 3194 stretching vibrations of (NH2). 3115 stretching vibration (NH). 3003 (C-H) stretching vibration of aromatic.1637 (C=N) stretching vibration. 1494,1449, (C=C) stretching vibration of aromatic. 686 stretching vibration of C-S. 503 stretching vibration for M-N, and 449 for M-S.
Compound (IVe) FTIR (cm-1): 3302, 3196 stretching vibrations of (NH2).3111 stretching vibration (NH). 3053 (C-H) stretching vibration of aromatic.1639 (C=N) stretching vibration. 1498,1450, (C=C) stretching vibration of aromatic. 690 stretching vibration of C-S. 505 stretching vibration for M-N, and 435 for M-S.
Nuclear magnetic resonance
The 1H NMR data of 4-amino-5-(phenyl) 4H-1,2,4-triazole-3-thiol and its complexes exhibited excellent solubility in DMSO. The proton nuclear magnetic resonance spectral data provided further validation for the composition of the complexes. The observed alterations are indicative of complexation, as the chemical shift of a molecule is largely dependent on its electrical environment [14,25-27].
Compound (III) 1HNMR (ppm): 5.83(singlet, 2H of NH2). 13.97 (singlet, 1H, of S-H) thiol. 7.51, 7.51, 7.72, 7.53, and 8.04, 8.05 (Multiplet signals result from the overlapping of non-equivalent aromatic protons, 6H, Ar-H).
Compound (IVa) 1HMNR (ppm): 3.98 (2H, s, NH2) (this peak is shifted to a lower field due to its attachment to the Nickel atom), 7.48-8.03 (5H, d,d, CH), 10.79 (1H, s, NH).
Compound (IVb) 1HMNR (ppm): 3.78 (2H, s, NH2) (this peak is shifted to a lower field due to its attachment to the Cobalt atom), 7.50-8.04 (5H, d,d, CH), 10.58 (1H, s, NH).
Compound (IVc) 1HMNR (ppm): 4.17 (2H, s, NH2) (this peak is shifted to a lower field due to its attachment to the Cadmium atom), 7.50-8.06 (5H, d,d, CH), 11.21 (1H, s, NH).
Compound (IVd) 1HMNR (ppm): 3.78 (2H, s, NH2) (this peak is shifted to a lower field due to its attachment to the Chromium atom), 7.52-8.06 (5H, d,d, CH), 10.99 (1H, s, NH).
Compound (IVe) 1HMNR (ppm): 4.36 (2H, s, NH2) (this peak is shifted to a lower field due to its attachment to the Zinc atom), 7.50-8.03 (5H, d,d, CH), 11.23 (1H, s, NH).
In vitro cytotoxicity evaluation
The synthesized compounds were evaluated against acetazolamide and cisplatin to assess their cytotoxic activity. The rationale for choosing these two drugs is that Acetazolamide serves as the benchmark for carbonic anhydrase inhibitors and has been well investigated for its potential as an antineoplastic agent. Multiple studies examined its efficacy and activity in countering neoplastic development. Cisplatin is an antineoplastic agent utilized for solid tumors, distinguished by the presence of a platinum metal ion in its chemical structure. Cisplatin, recognized for its known antineoplastic efficacy, serves as a crucial reference chemical for investigating the cytotoxic characteristics of synthetic agents. Tab. 2. and Fig. 2. display the IC50 values of the synthesized compounds and standards. Fig. 3. displays the cytotoxicity activity evaluation of triazole-metal complexes (IVa-IVe) on MCF10, while Fig. 4. on MCF7, respectively.
| Compound | MCF7 Results | MCF10a Results | ||
|---|---|---|---|---|
| IC50(μM) ± SD | p-value | IC50(μM) ± SD | p-value | |
| Acetazolamide | 145.10 ± 1.58 | Standard | 71.80 ± 0.566 | Standard |
| Cisplatin | 20.68 ± 0.776 | Standard | 36.24 ± 1.036 | Standard |
| IVa | 159.00 ± 4.44 | 10.61 * (0.0378) 8.96 ** 0.0001 |
1295.03 ± 4.86 | 22.93 ** (0.0001) 18.57 ** (0.0001) |
| IVb | 103.27 ± 2.30 | 12.76 ** (0.0056) 8.32 ** (0.0001) |
3482.71 ± 3.76 | 52.85 ** (0.0001) 28.69 ** (0.0001 |
| IVc | 28.45 ± 2.34 | 8.94** (0.0001) 5.26* (0.0398) |
138.62 ± 3.60 | 38.44 ** (0.0001) 25.75 ** (0.0001) |
| IVd | 90.92 ± 5.16 | 9.47 ** (0.0001) 7.37 ** (0.0001) |
1333.23 ± 2.86 | 48.61 ** (0.0001) 28.96 ** (0.0001) |
| IVe | 52.57 ± 4.72 | 8.36 ** (0.0001) 6.46 ** (0.0001) |
316.50 ± 4.12 | 18.26 ** (0.0001) 15.02 ** (0.0001) |
| * (P<0.05), ** (P<0.01). * Significant, ** Highly significant |
||||
Tab. 2. IC50(µM) values for the tested compounds. The values are represented by the mean ± SD of triplicate measurements. IC50: Half-maximal inhibitory concentration. MCF7: a human breast cancer cell line with estrogen, progesterone, and glucocorticoid receptors. MCF10: a non-tumorigenic epithelial cell line. (IVa-IVe) The synthesized triazole-metal complexes. (*) and (**): there is a considerable variation compared to Acetazolamide and Cisplatin, respectively.

Fig. 2. IC50 (µM) values for the tested compounds. The values are represented by the mean ± SD of triplicate measurements. IC50: Half-maximal inhibitory concentration. MCF7: a human breast cancer cell line with estrogen, progesterone, and glucocorticoid receptors. MCF10: a non-tumorigenic epithelial cell line. (IVa-IVe) The synthesized triazole-metal complexes.

Fig. 3. Illustrates a cytotoxicity assessment evaluating the effects of several agents on MCF10a cells. A) Inhibitory activity of Acetazolamide, B) Inhibitory activity of Cisplatin, C) Inhibitory activity of Compound IVa, D) Inhibitory activity of Compound IVb, E) Inhibitory activity of Compound IVc, F) Inhibitory activity of Compound IVd, G) Inhibitory activity of Compound IVe.
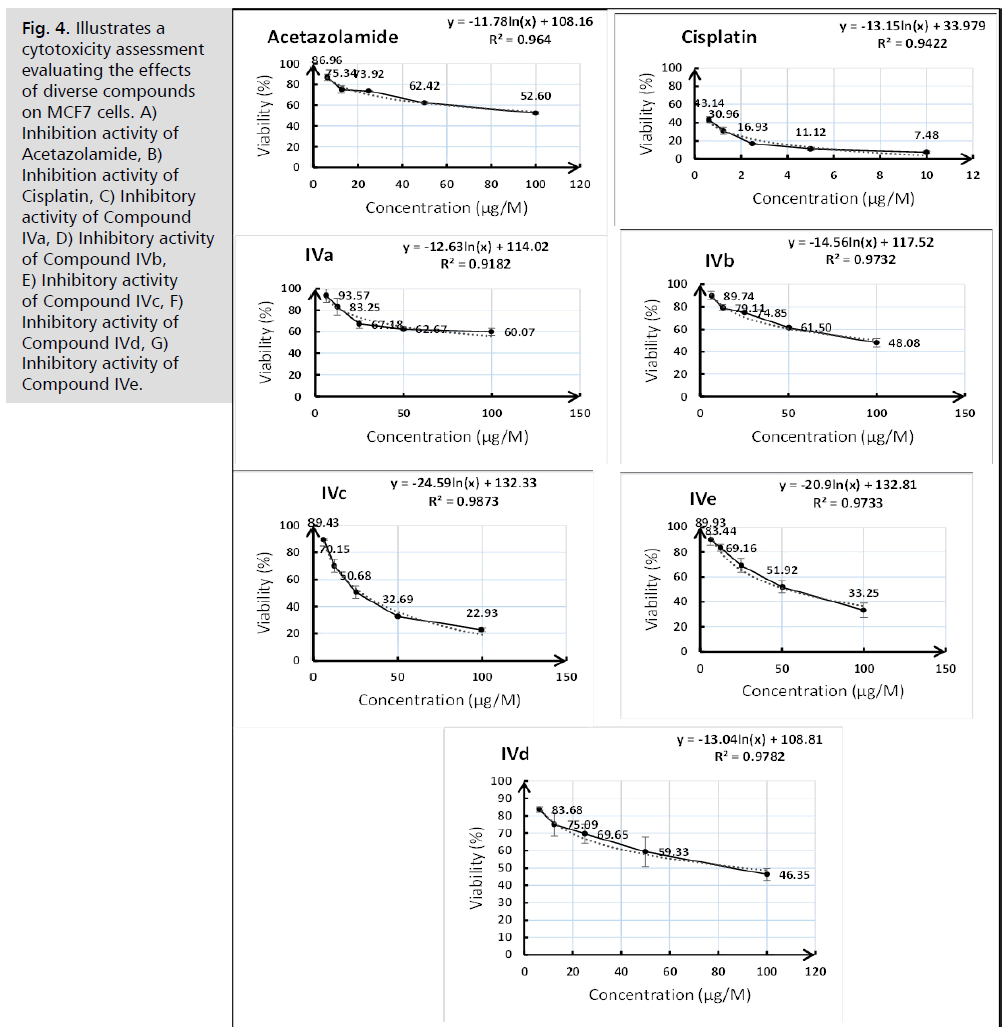
Fig. 4. Illustrates a cytotoxicity assessment evaluating the effects of diverse compounds on MCF7 cells. A) Inhibition activity of Acetazolamide, B) Inhibition activity of Cisplatin, C) Inhibitory activity of Compound IVa, D) Inhibitory activity of Compound IVb, E) Inhibitory activity of Compound IVc, F) Inhibitory activity of Compound IVd, G) Inhibitory activity of Compound IVe.
Discussion
Regarding infra-red spectroscopy, the FT-IR spectra of the synthesized compounds provided strong evidence for their structural features and functional group identification. For Compound I (Bezohydrazide), the N-H stretching vibrations at 3414 cm-1 and 3300 cm-1 , along with the C=O stretching band at 1660 cm-1, confirmed the presence of the amide group. In Compound II (Potassium 2-benzoylhydrazine-1-carbodithioate), the NH stretching vibrations at 3327 cm-1 and 3157 cm-1 and the C=O stretching at 1641 cm-1 confirmed the secondary amide and amine functional groups. Additionally, the C-S stretching band at 630 cm-1 supported the presence of the carbodithioate group, a key feature of this compound. The FTIR spectrum of this ligand (L) showed some characteristic stretching bands at: 3250 cm-1 and 3213 cm-1, 2736 cm-1, 1645 cm-1, 673 cm-1 assigned to NH2, S-H, C=N of triazole ring, and the last one is for stretching of C-S bond, respectively. The tautomeric form may manifest within the triazole ring, as illustrated in Fig. 5. It is plausible to anticipate the deprotonation of the compound (III) molecule prior to complexation; the total absence of the band corresponding to υ(S-H) in the spectra of the complexes unequivocally corroborates this perspective. Upon deprotonation, the ligand can coordinate with the metal ion through either the nitrogen or sulfur atom of the thioamid group. Bonding at the sulfur atom is more advantageous since it leads to the formation of a stable five-membered chelate.
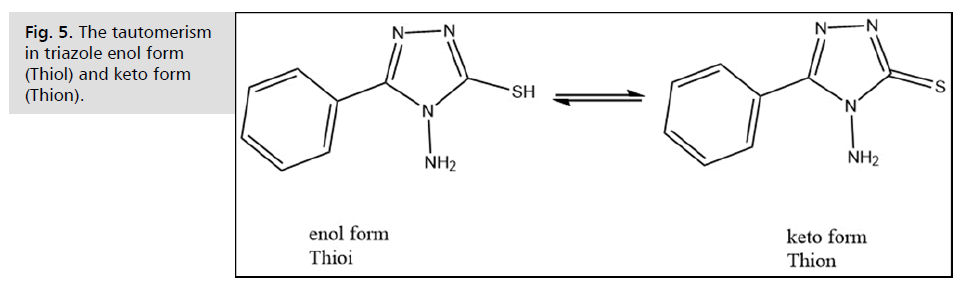
Fig. 5. The tautomerism in triazole enol form (Thiol) and keto form (Thion).
The unique instance is that the υ(C = N) of complexes (IVa-IVe) was observed to be shifted to a lower wavenumber in comparison to compound (III), indicating coordination with the ligand. The NH2 band frequencies were altered as a result of complexation. The S-H band of the ligand disappeared upon complexation. The bands of C-S changed to higher frequencies due to the complexation of the metal ion with the ligand via sulfate, and the band of C=N also occurred.
The frequency is lowered due to complexation, while other bands, such as C=C (1494-1450 cm-1) and C-H aromatic (3050-2997 cm-1), did not exhibit any shifts as they do not participate in the complexation.
Regarding 1H NMR, the 1H NMR data for compound III (4-amino-5-phenyl 4-H 1,2,4-triazole 3 thiol) and its five triazole complexes provided further evidence for complexation, indicated by the downfield shift of the NH2 signal due to metal attachment, compared to compound III. Additionally, the SH signal at 13.97 ppm in the ligand was absent, while a new NH signal appeared in the range of 10-11.5 ppm, which was not present in compound III prior to complexation. The aromatic proton is present at a signal of 7.5-8.06 ppm in compound (III) and all five complexes, as it does not engage in complexation.
Regarding to in-vitro cytotoxicity evaluation
The cytotoxic activities of the synthesized triazole-metal complexes (IVa–IVe) were assessed against MCF-7 and MCF-10a cell lines, using acetazolamide and cisplatin as reference standards. The IC50 values demonstrated a variable but generally significant cytotoxic effect against MCF-7 cells, with Compound IVc (Cd2+ complex) showing the lowest IC50 (28.45 ± 2.34 μM) among the tested complexes, approaching the potency of cisplatin (20.68 ± 0.78 μM). This suggests that coordination with cadmium enhances the cytotoxic potential of the ligand core, possibly through increased cellular uptake or enhanced affinity for intracellular targets.
All complexes displayed significantly reduced cytotoxicity toward MCF-10a cells, with IC50 values several-fold higher than in MCF-7, particularly for IVb (Co2+) and IVd (Cr3+), which had IC50 values exceeding 1300 μM. This pronounced selectivity indicates a promising therapeutic window and suggests potential for further development of these complexes as anticancer agents.
Statistical analysis revealed that the differences in cytotoxicity between treated and standard groups were significant (p<0.01) in most comparisons, supporting the robustness of the results. Overall, the biological evaluation demonstrates that triazole-metal complexation significantly influences cytotoxic behavior, with IVc and IVe exhibiting the most favorable balance between potency and selectivity.
Conclusion
The successful synthesis of triazole-based ligands and their metal complexes (IVa–IVe) was confirmed through FT-IR and 1H NMR spectroscopy, which supported the proposed structures and metal coordination. The biological evaluation revealed that several complexes, particularly IVc and IVe, exhibited notable cytotoxicity against MCF-7 cancer cells while showing significantly lower toxicity toward normal MCF-10a cells, indicating a favourable selectivity profile. These findings suggest that metal complexation enhances the anticancer potential of the triazole scaffold and supports further investigation of these compounds as promising chemotherapeutic candidates.
Acknowledgments
The authors would like to express their gratitude to the Faculty of Pharmacy at Universitas of Kufa, Najaf, Iraq, for providing the facilities and equipment that supported this research.
Conflict Of Interest Statement
The authors declared no conflict of interest in the manuscript.
References
- Naji EM, Naser NH, Hussein SA. In silico study, synthesis, and antineoplastic evaluation of thiazole-based sulfonamide derivatives and their silver complexes with expected carbonic anhydrase inhibitory activity. J Med Life. 2023;16(12):1857-1864.
- Slman DK, Satar HA, Ketan ZA, et al. Heterocyclic compounds: a study of its biological activity. Al-Nahrain J Sci. 2024;27(5):19-24.
- Ameziane El Hassani I, Rouzi K, Ameziane El Hassani A, et al. Recent developments towards the synthesis of triazole derivatives: A review. Organics. 2024;5(4):450-471.
- Bachay IA, Naser NH. Synthesis and in silico study of new acetazolamide derivatives incorporating a 1,2,4-triazole moiety as potential carbonic anhydrase inhibitors and anti-cancer agents. Trop J Nat Prod Res. 2024;8(5):7207-7212.
- Abbas ZK, Naser NH, Atiya RN. In silico study of novel sulfonamide derivatives bearing a 1,2,4-triazole moiety act as carbonic anhydrase inhibitors with promising anti-cancer activity. Pol Merkuriusz Lek. 2023;51(5):527-532.
- Atin MM, Matin P, Rahman MR, et al. Triazoles and their derivatives: Chemistry, synthesis, and therapeutic applications. Front Mol Biosci. 2022;9:864286.
- Rani S, Teotia S, Nain S. Recent advancements and biological activities of triazole derivatives: A short review. Pharm Chem J. 2024;57(12):1909-1917.
- Abdulameer JH, Alias MF. Heavy metal complexes of 1,2,3-triazole derivative: synthesis, characterization, and cytotoxicity appraisal against breast cancer cell lines (MDA-MB-231). Baghdad Sci J. 2022;19:1410-1416.
- Santosh R, Selvam MK, Kanekar SU, et al. Synthesis, characterization, antibacterial and antioxidant studies of some heterocyclic compounds from triazole-linked chalcone derivatives. Chem Select. 2018;3(23):6338-6343.
- Timur İ, Kocyigit ÜM, Dastan T, et al. In vitro cytotoxic and in vivo antitumoral activities of some aminomethyl derivatives of 2,4-dihydro-3H-1,2,4-triazole-3-thiones—Evaluation of their acetylcholinesterase and carbonic anhydrase enzymes inhibition profiles. J Biochem Mol Toxicol. 2019;33(1):e22239.
- Doohee AN, Naser NH, Karrar AG. Molecular docking, synthesis, and biological activity of new diclofenac derivatives incorporating 1,2,4-triazole ring as promising antibacterial agents. Iraqi J Pharm Sci. 2025;34(1):165-175.
- Rajavelu K, Subaraja M, Rajakumar P. Synthesis, optical properties, and antioxidant and anticancer activity of benzoheterazole dendrimers with triazole bridging unit. New J Chem. 2018;42(5):3282-3292.
- El Azab IH, El-Sheshtawy HS, Bakr RB, et al. New 1,2,3-triazole-containing hybrids as antitumor candidates: design, click reaction synthesis, DFT calculations, and molecular docking study. Molecules. 2021;26(3):708.
- Haddad R, Yousif E, Ahmed A. Synthesis and characterization of transition metal complexes of 4-amino-5-pyridyl-4H-1,2,4-triazole-3-thiol. SpringerPlus. 2013;2:510.
- Kovács A, Varga Z. Metal-ligand interactions in complexes of cyclen-based ligands with Bi and Ac. Struct Chem. 2021;32(5):1719-1731.
- Yousif EA, Hameed AS, Ameer AA. Synthesis and characterization of complexes of some transition metals with 2-amino-5-(4-hexyloxyphenyl)-1,3,4-thiadiazole. Al-Nahrain J Sci. 2005;8(1):9-11.
- Dhale PC, Ubale PA, Sonawane KD, et al. New triazole-based Schiff base ligands and their Co(II) and Ni(II) complexes as biological potent molecules: chemical preparation, structural elucidation, and biological studies. Results Chem. 2023;6:101155.
- Zhang LX, Zhang AJ, Lei XX, et al. 4-Amino-3-(1,2,3,4,5-pentahydroxypentyl)-1,2,4-1H-triazole-5(4H)-thione. Acta Crystallogr E Struct Rep Online. 2004;60(4):o613-o615.
- Volpi G, Zago S, Rabezzana R, et al. N-Based polydentate ligands and corresponding Zn(II) complexes: a structural and spectroscopic study. Inorganics. 2023;11(11):435.
- Majeed A, Alabdeen K. Synthesis and characterization of new thio-triazole ligand and complexes with selected metals. J Pharm Bio Sci. 2012;4(5):9-14.
- Singh AK, Kandel KR. Synthesis of triazole derivative: [4-(benzylideneamino)-5-phenyl-4H-1,2,4-triazole-3-thiol]. J Nepal Chem Soc. 2012;30:174-177.
- Sarafroz M, Khatoon Y, Ahmad N, et al. Synthesis, characterization and anticonvulsant activity of novel fused 1, 2, 4-triazolo-1, 3, 4-thiadiazoles. Oriental J Chem. 2019;35(1):64-69.
- Helwig JT, Council KA. Statistical analysis system user's guide. Carey, NC, SAS Institute Inc. 1979:221-236.
- Silverstein RM, Webster FX, Kiemle DJ. Spectrometric identification of organic compounds. 7th ed. New York: John Wiley & Sons; 2005.
- Ibraheem H, Adel H, Ahmed A, et al. Synthesis, characterization and antimicrobial activity of some metal ions with 2-thioacetic-5-phenyl-1,3,4-oxadiazole. Al-Nahrain J Sci. 2010;13(1):43-47.
- Hassan SM, Al-Jaf ANA, Hussien YA, et al. Effect of new synthesized compounds of 2-thiouracil sulfonamide derivatives against colon and liver carcinoma cells “in vitro study". Int J Pharm Res. 2020;12(4):2012-2016.
- Jabber EJ, Alzayd AM, Jawad MJ, et al. Synergistic effect of oxytetracycline as a combination treatment with Carboplatin on MCF-7 breast cancer cell line. Braz J Vet Res Anim Sci. 2022;59:1-9.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Israa A. Bachay1* and Noor H. Naser22Department of Pharmaceutical Chemistry, College of Pharmacy, Al-Zhraa University for Women, Karbala, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.