Research - (2024) Volume 19, Issue 1
Prevention of hypotention after spinal anesthesia in cesarean section â A prospective randomized comparative clinical study on ephedrine vs. norepinephrine
Sami R. Hasan1,2, Afraa Ibrahim1, Talib Razq M3 and Hayder A. Fawzi4*Received: 02-Jan-2024, Manuscript No. gpmp-24-124498; Editor assigned: 04-Jan-2024, Pre QC No. P-124498; Reviewed: 16-Jan-2024, QC No. Q-124498; Revised: 22-Jan-2024, Manuscript No. R-124498; Published: 29-Jan-2024
Abstract
Background: Ephedrine (EP) and Norepinephrine (NE) considered an option to maintain maternal blood pressure during Spinal Anesthesia (SA) since hypotension is considered a common consequence of SA. The aim of the current study is the assessment of the maternal and neonatal safety and efficacy of NE compared to ephedrine in treatment of SA induced hypotension in cesarian delivery.
Methods: A prospective, randomized comparative clinical study, that involved 100 participants, they were divided into two groups: Group N: received NE (10 μg/ml). Group E: received EP (5 mg/ml). The research was conducted at tertiary Teaching Hospital, and it lasted from February 2021 until October 2022.
Results: Overall, the incidence of hypotension was significantly lower in NE group, and when assessed at various time periods (for 60 minutes), it was significant lower only at 10 minutes. The use of NE was associated with reduction in the risk of hypotension (OR: 0.439) compared to the use of EP.
Conclusion: NE is an effective and safe vasopressor in preventing postanesthetic hypotension in SA for CS devilries, with less hypotensive episodes, better hemodynamic maternal effect, low incidence of nausea, and better neonatal umbilical cord blood gases compared to EP.
Keywords
Anesthesia; Spinal; Vasopressor; Hypotension
Introduction
Isolation of local anesthetics, with cocaine as the pioneering example, led to the creation of regional anesthesia. Spinal Anesthesia (SA) was the first regional anesthetic technique used, and the first operation utilizing the method was done in 1898 by German surgeon August Bier [1]. For most surgeries below the neck, neuraxial anesthesia is used either alone or in conjunction with general anesthetic. Surgical operations below the umbilicus benefit greatly from SA because of its ability to access the lower abdominal, pelvic, perineal, and lower limb regions [2].
Without medication prophylaxis, post-spinal anesthetic hypotension can occur in as many as 70% of women undergoing cesarean birth, making it one of the most prevalent concerns [3]. Nausea, vomiting, and maybe even harm to the fetus are all possible side effects of this. Taking preventative steps such as a fluid preload, a lateral tilt, or a dose of a vasoactive drug are all helpful [4]. EP is a popular choice among vasoactive medicines. EP's subtle onset of action and extended duration, however, make precise titration of blood pressure challenging [5]. EP crosses the placenta quickly, and it can cause fetal tachycardia to emerge out suddenly, which can lead to fetal acidosis [6].
Preventing post-spinal hypotension using NE, a powerful a-adrenergic receptor agonist with a moderate impact on the beta-adrenergic receptor, is a relatively new development. As a result, NE may be preferable to epinephrine for the purpose of maintaining parturient's blood pressure during cesarean delivery as it has fewer adverse effects on Heart Rate (HR) [7].
Although EP is routinely used as a vasopressor to counteract post spinal hypotension [5], there's been little research comparing it to NE.
NE's quick onset and brief duration of effect make it more ideal for infusion [8], and a research showed that utilizing a bolus of NE to maintain parturients' blood pressure during cesarean delivery produced greater hemodynamic stability than using an EP bolus [9].
The current study examined intermittent injection of both medications, while most of the study focused on infusion, its not always available in resources limited countries like Iraq, and in addition the use of pump require training and additional cost to purchase and maintain them. Thus, the use of intermittent injection is attractive for its ease and low cost. The aim of the current study is the assessment of the maternal and neonatal safety and efficacy of NE compared to EP in treatment of SA induced hypotension in cesarian delivery.
Materials and Methods
Study design
A prospective, randomized single blinded clinical study, which involved 100 participants, they were divided into two groups:
Group N: Received NE (10 μg/ml).
Group E: Received EP (5 mg/ml).
When the MAP dropped below 60 mmHg, an intervention was given. Hypotension was defined as a decline of >20 percent from the original value of MAP.
Study setting
The research was conducted at Habboubi Teaching Hospital in Nasiriyah Governate in Iraq, and it lasted from February 2021 until October 2022.
Study groups
EP Group: After giving them 1 mL of intravenous EP at a dosage of 5 mg/mL, their MAP levels were checked five minutes later. If the blood pressure has not yet reached 60 mmHg, administer the same dose no more than three times at intervals of five minutes.
NE Group: They received a 10g/1mL intravenous bolus of NE, which was made as follows: from a 4 mg/2 ml vial, 1ml was taken and diluted with 20 ml of saline (100 g/ml), and then 1ml was taken and diluted with 10 ml to produce the final solution with 10 g/ml. After two minutes, the MAP was assessed. The same dose should only be given a maximum of three times, separated by two minutes, if it has not yet reached 60 mmHg.
This procedure was repeated for every MAP that dropped below 60 mmHg while the patient was under anesthesia. If the MAP in either group did not reach 60 mmHg after three injections, another strategy should have been used to raise it. A peripheral venous catheter was used to provide vasopressors.
Randomization
A computer-generated pattern was used to randomly assign patients in a 1:1 ratio to the NE group or EP group.
Sample size
Based on previous study in which EP at a dose of 5 – 10 mg the incidence of post anaesthesia hypotension was 11.4% [10], while for epinephrine it was 37.5% [11]. After performing sample size calculations at type I error 0.05 and type II error 0.2 the calculated sample was 49 for each group, so we choose 50 for each group.
Inclusion criteria
ASA I-II
Age between 18 – 40 years
Full-term pregnancy
Elective caesarian surgery
Exclusion criteria
Lack of cooperation
Patients refused to be included in the study
Patients with spinal deformities
Patients with height less than 150 cm
Morbid obesity (BMI ≥ 35 kg/m2)
Coagulopathy, or on anticoagulation therapy
History of allergies to any medication used in the study
Anesthetic procedure
Before administering anesthesia, baseline measures of the Heart Rate (HR) and non-invasive Systolic Blood Pressure (SBP) (measured at the upper left limb) were made five minutes apart. An IV catheter that had been inserted in the upper extremities was used to preload 10 mL/kg of lactated Ringer's solution prior to spinal injection, and the infusion rate was then decreased to a maintenance rate. SA was carried out in the appropriate lateral position by experienced anesthetists. After skin sterilisation and localized lidocaine injection at 2%.
In our trial, the drug was started to be administered at the moment of the spinal injection. Patients were placed supine with a 15° left lateral table tilt after SA. Prior to making an incision for surgery, the sixth thoracic dermatomal level was the lowest tolerable level according to bilateral pinprick testing of dermatomal levels of analgesia. SBP and HR were monitored for 30 minutes after spinal injection at intervals of every 2 minutes.
In patients with weak uterine contractions, an extra 250 mg of carboprost tromethamine was administered intramuscularly in addition to the standard dosage of oxytocin (Syntocin®)—10 milliunits with direct infusion—and 20 milliunits. A crystalloid solution in the amount of 500–1000 ml is given. The infant radiant warmer was instantly switched over to the newborns.
Preoperatively, all patients receiving regional anesthesia gave 0.02 mg/kg of intra-venous sedative with midazolam. SA was performed in the L3-L4 intervertebral area using a midline approach, a 25-gauge Quincke needle, and a downward-facing bevel. 2.0 mL (10 mg) of intrathecal penetration of the needle was verified with free flow of CSF by injecting 0.3 milliliters of hyperbaric bupivacaine per second for 60 seconds into the affected area [12]. For breakthrough pain 50 mg tramadol IV injection was used.
Ethical consideration
The Iraqi Ministry of Health gave approval for this study (ID#: 270/31/5/2021; number: 0431570) [13]. Written consentient were taken from all those who participated in the study.
Statistical analysis
All analysis was carried out using Graph Pad prism 9.01, for analyzing continuous variables (all followed normal distribution) independent t-test was used, for categorical variables chi square test used. Binary logistic regression used for risk assessment of hypotension and data reported as Odd Ratio (OR) and its 95% Confidence Interval (CI). Level of significance was 0.05 and all p-value was two tailed.
Results
Demographic and maternal characteristics
The study included 100 participants, which were divided into two groups, the first group received EP (50 patients), and the other group received NE (50 patients). There was no significant difference in age, BMI, gravida, and gestational age, as illustrated by Tab. 1.
| Variables | Ephedrine | Norepinephrine | p-value |
|---|---|---|---|
| Number | 50 | 50 | - |
| Age (y), mean ± SD | 28.22 ± 6.31 | 30.46 ± 6.20 | 0.076 |
| BMI (kg/m2), mean ± SD | 28.47 ± 3.85 | 28.68 ± 4.19 | 0.795 |
| Gravida, n (%) | |||
| Primigravida | 16 (32.0%) | 13 (26.0%) | 0.509 |
| Pluripara | 34 (68.0%) | 37 (74.0%) | |
| Gestational age (weeks), mean ± SD | 38.30 ± 0.95 | 38.16 ± 1.08 | 0.493 |
Tab. 1. Assessment of demographic and maternal characteristics.
Assessment of hemodynamic stability of the mothers
There was no significant difference in Systolic Blood Pressure (SBP) at baseline and after 5 minutes, afterward SBP was significantly higher in NE at 10, 15, 30, and 60 minutes, as illustrated by table S1 and Fig. 1. There was no significant difference in Diastolic Blood Pressure (DBP) from baseline till 60 minutes of follow-up, as illustrated by Tab. S2. and Fig. 2. Only after 15 minutes Mean arterial Blood Pressure (MBP) was significantly higher in NE, while at the rest of the time period no significant difference was observed, as illustrated by table S3 and Fig. 3. Heart rate was significantly lower in NE group from 5 minutes till the end of 60 minutes of follow-up, as illustrated by Tab. S4. and Fig. 4. There was no significant difference in SPO between both groups at all time periods, as illustrated by table S5.
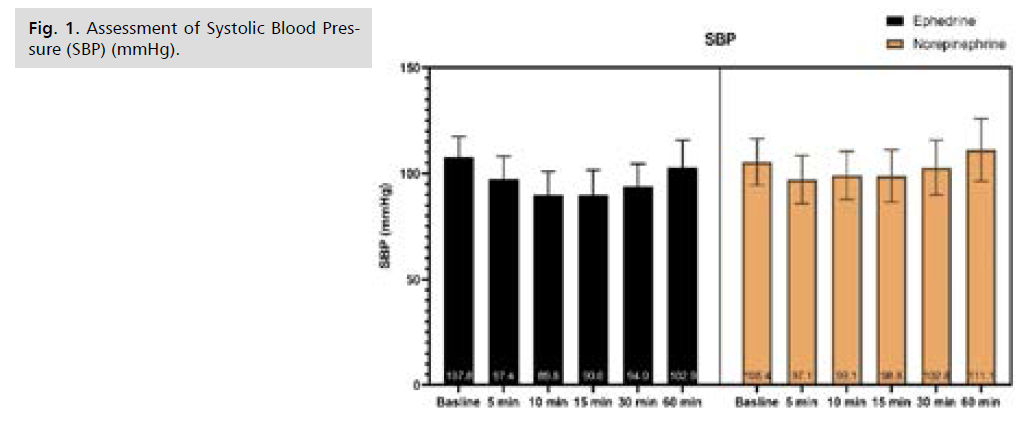
Fig 1. Assessment of Systolic Blood Pressure (SBP) (mmHg).
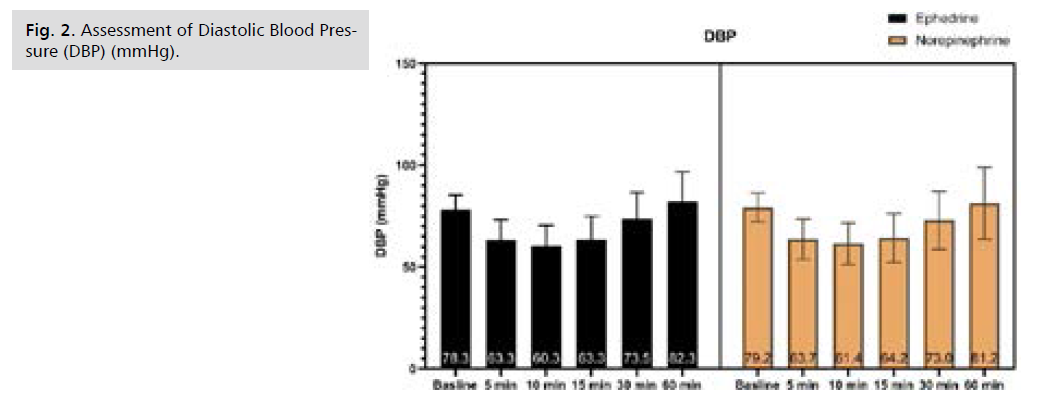
Fig 2. Assessment of Diastolic Blood Pressure (DBP) (mmHg).
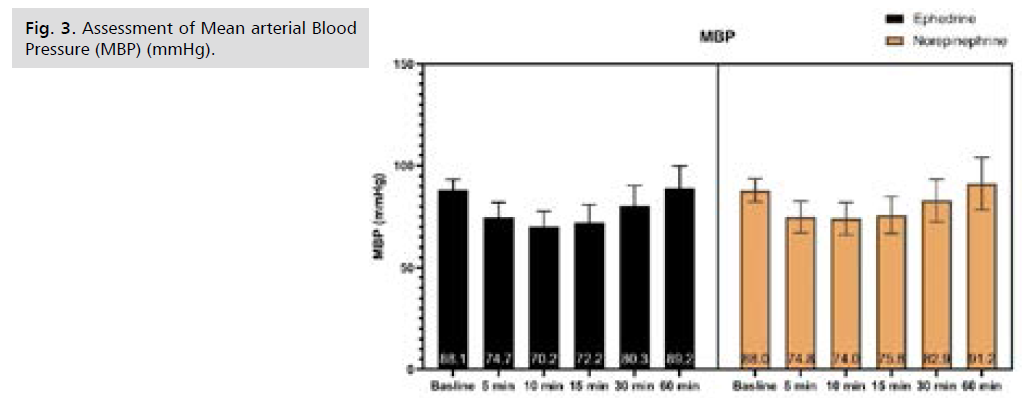
Fig 3. Assessment of Mean arterial Blood Pressure (MBP) (mmHg).
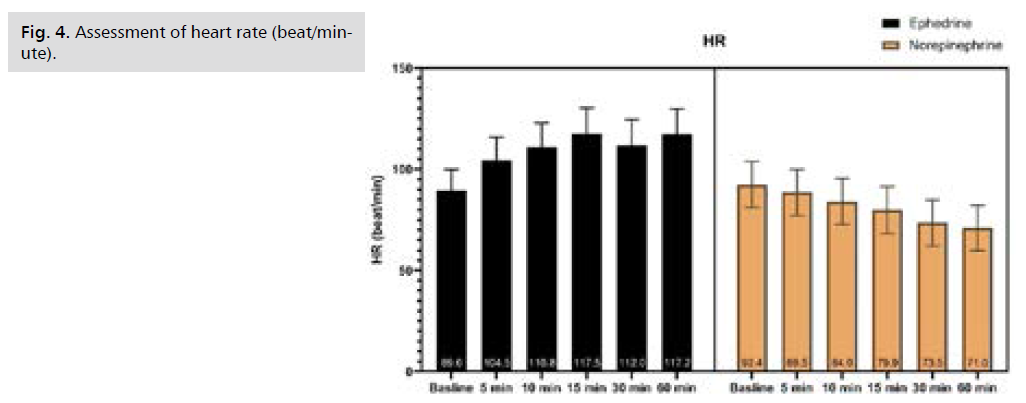
Fig 4. Assessment of heart rate (beat/minute).
Assessment of incidence of hypotension and need for rescue therapy
Overall, the incidence of hypotension was significantly lower in NE group, and when assessed at various time periods, it was significant lower only at 10 minutes, as illustrated by table S6. The use of NE was associated with reduction in the risk of hypotension (OR: 0.439) compared to the use of EP, which indicate that NE reduce the risk of hypotension by 56.1% (absolute relative risk reduction=1.0-0.439 × 100%), as illustrated by Tab. 2.
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| Use of NE vs. EP | 0.439 | 0.196 – 0.984 | 0.046 |
Tab. 2. Effect of medication of risk of hypotension.
There was no significant difference in the need for rescue analgesia between both groups, as illustrated by Tab. S7.
Neonatal outcomes
There was no significant difference in mean APPGAR score after both 1 and 5 minutes of neonatal delivery. All neonatal arterial blood gases did not show significant difference between both NE and EP, as illustrated by Tab. 3.
| Time | Ephedrine | Norepinephrine | p-value |
|---|---|---|---|
| Number | 50 | 50 | - |
| 1 minute | 8.12 ± 0.82 | 7.84 ± 0.84 | 0.096 |
| 5 minutes | 9.04 ± 0.83 | 9.08 ± 0.72 | 0.798 |
| pH | 7.33 ± 0.02 | 7.38 ± 0.02 | <0.001 |
| HCO3 (mEq/liter) | 23.60 ± 1.11 | 23.94 ± 1.11 | 0.129 |
| PaO2 (mm Hg) | 25.2 ± 2.7 | 25.3 ± 2.8 | 0.822 |
| PaCO2 (mm Hg) | 40.52 ± 3.06 | 39.56 ± 2.88 | 0.108 |
| SpO2 (%) | 94.74 ± 1.59 | 94.52 ± 1.51 | 0.468 |
Tab. 3. Assessment of neonatal and arterial blood gases of the neonate.
Adverse effect
Nausea was significantly lower in NE compared to EP, while no significant difference between both groups in the incidence of vomiting, shivering, and head-ache, as illustrated by Tab. 4.
| Time | Ephedrine | Norepinephrine | p-value |
|---|---|---|---|
| Number | 50 | 50 | - |
| Nausea | 17 (34.0%) | 8 (16.0%) | 0.038 |
| Vomiting | 10 (20.0%) | 4 (8.0%) | 0.084 |
| Shivering | 6 (12.0%) | 2 (4.0%) | 0.269 |
| Headache | 4 (8.0%) | 5 (10.0%) | 0.999 |
Tab. 4. Comparison of adverse effects.
Discussion
Main results hypotension and hemodynamic stability
In the present study the incidence of postanesthetic hypotension was significantly lower in women received NE compared to EP (34%, vs. 54%, p-value=0.044), with absolute risk reduction of 56.1% in the incidence of hypotension, this covers the incidence at any time period. In more detailed assessment after 10 minutes the incidence of hypotension was significantly lower in NE compared to EP (30, vs. 52%, p-value=0.025). For the rest of the time periods there were no significant difference between both groups, despite the incidence of hypotension is lower in NE in most of the time periods. In this study, we chose these five times (5, 10, 15, 30, and 60 minutes) when the blood flow is usually unstable, this instability after SA was started is related to the systemic vascular resistance went down, but the cardiac output, heart rate, and stroke volume all went up by a small amount [8].
In term of hemodynamic stability, systolic blood pressure was significantly higher in NE compared to EP (at 10, 15, 30, and 60 minutes), diastolic blood pressure was not different between both groups at all time periods, mean blood pressure was significantly higher in NE compared to EP (at 10 and 15 minutes). Heart rate was significantly lower in NE compared to EP. This indicates that NE offer better safety profile compared to EP, which is added to its better outcome to prevent hypotension in post-SA procedures. In terms of need for rescue analgesia, there was no significant difference between both NE and EP in that aspect, of notice the numerical value was lower in NE compared to EP (12% vs. 6%, p-value=0.487), however it did not reach a statistical significance. In 2015, Ngan Kee, et al. were the first to conclude that NE was used to preserve blood pressure constant throughout the experiment during SA during cesarean deliveries. By using computer-controlled infusion, they showed that NE raised the heart rate and cardiac output more than phenylephrine did but had the same effect on lowering blood pressure [14].
In Hassani, et al. study, a RCT (Randomized Clinical Trial), done in Iran, 56 hypertensive patients were divided into two groups (each 28 patients) according to antihypertensive drug (either NE or EP) all patients received SA (SA) and were followed up prospectively. The incidence of hypotension was significantly lower in NE compared to EP, MAP was significantly higher in NE compared to EP, HR was significantly lower in NE compared to EP, and the number of rescue boluses of vasopressors during the SA was significantly lower in NE compared to EP [15]. These findings were in agreement with the current study. In Wang, et al. study that examined the bolus doses of NE (4 μg) and EP (4 mg) for hypotension prevention in CS, they found that HR was significantly higher in NE [16].
The hypotensive impact of SA after cesarean birth was studied by Ali Elnabtity and colleagues, who compared intravenous boluses of EP and NE as a treatment. They demonstrated that NE was superior to EP in terms of maintaining blood flow in the uterine artery and maternal blood pressure. Additionally, NE was associated with fewer episodes of hypotension and hypertension, as well as a lower incidence of bradycardia and tachycardia. Furthermore, it needed a lower total number of boluses [9]. Which is in agreement with the current study? A study by Abd Elraziq, et al., SBP was significantly higher in NE compared to EP at 2 and 4 minutes afterwards till 1 hour of follow-up no significant difference was found, HR was significantly lower in NE compared to EP at 4-, 6-, 8-, and 10-minutes afterwards till 1 hour of follow-up no significant difference was found [17]. In Fan, et al. study, that examined 0.05 mg•kg-1•min-1 NE infusion for 30 minutes, compared to 0.15 mg/kg EP bolus, in 190 CS women hypotension was lower in NE compared to EP (29.5% vs. 44.9%, with 0.51, P-value=0.034), SBP decrease was significantly higher in EP compared to NE, incidence of tachycardia was significantly in EP [18]. Which is in agreement with the current study? Another study shown that NE is efficient in maintaining systolic blood pressure with a drop in heart rate, which is beneficial for individuals who suffer from coronary artery disease [19]. EP stimulates the sympathetic nervous system and has potent inotropic and chronotropic effects on the heart. Its action might be either directly (by acting as an agonist for alpha or beta receptors) or indirectly (catecholamine, namely NE release). It lowers afterload, raises cardiac output, raises blood pressure and heart rate, and produces modest arteriolar constriction [9]. The vasoconstrictive impact of EP is reduced with continued use of the drug. It have slow onset of action [9]. EP has been linked to an increased risk of tachycardia, tachyphylaxis, and hypertension [20]. NE has both beta- and alpha-adrenergic effects (weak beta-adrenergic and potent alpha -adrenergic receptor agonist [9]), which could lead to a higher heart rate and cardiac output than EP and a lower chance of bradycardia [8]. It causes an arterial and venous vasoconstriction and improves venous return and cardiac preload [9]. Several studies were in agreement with the current study findings; in which NE offer better hemodynamic control than EP.
Because anesthesiologists typically use lumbar anesthesia to diffuse local anesthetics in order to achieve spinal nerve block during cesarean section, this practice can cause hypotension manifestations like decreased cardiac blood volume and vascular dilation in the anesthesia area. This is an urgent problem that needs to be solved in the field of obstetrics and gynecology [21]. In a study by Huang, et al., that examine pregnant women that undergone CS with SA, two groups EP (40 women) and NE (40 women). They reported no significant differences in heart rate, while the SBP and DBP were significantly higher in NE compared to EP. Which is to the contrary to our findings in which no significant difference in SBP and DBP for both groups (this could be explained by the mode of use of NE in which both used as intermittent in our study while for Huang, et al. they used continues infusion for NE) [22]. NE is a strong -receptor agonist that has a clear effect on skin, mucosal, and glomerular blood vessels by making them constrict. Overall, if blood pressure is too high, it can stimulate the vagus nerve reflexively, like EP, and slow the heart rate. It can also cause tissues to not get enough blood, which can cause hypoxia and acidosis [23]. In Vallejo, et al. study, an open label randomized trial, they examined the prophylactic use of both NE and EP in CS delivery, they found no significant difference in hemodynamic parameters (HR, BP, and cardiac output) and number of recure vasopressors medication [24]. In this study the authors used a fixed-rate infusion which is a different approach than used by [14] which used a computer-controlled closed-loop feedback system to administer and titrate the vasopressors with CO as the primary outcome, and in the current study and [9] which used an intermittent bolus injection for controlling hypotension. These different led to different doses used for various studies. Also, in the [9,14] an estimate of a potency ratio of 20:1 was used, while in [24] they used potency ratio of 2:1. Last this Vallejo, et al. defined hypotension as the requirement for rescue bolus intervention, which is different from our study >20% reduction from the initial value of MAP [24]. EP is a drug that is often used to prevent and treat low blood pressure after lumbar anesthesia for a cesarean section. It can relax smooth bronchial muscles and stimulate the central nervous system, which causes the coronary arteries and cerebrovascular system to expand and the cardiac output to rise [25].
Neonatal outcome
SA is the method of choice for a planned CS, but it causes low blood pressure in the vast majority of women if nothing is done to stop it. Spinal hypotension in this situation can cause a decrease in blood flow to the uterus and placenta, fetal acidosis, and, in rare cases, a collapse of the heart and lungs [26]. In the present study the pH of neonatal was significantly lower in EP compared to NE (7.33 ± 0.02 vs. 7.38 ± 0.02), while the rest of Neonatal umbilical blood gas (NUBG) did not show significant difference between both EP and NE (HCO3, PaO2, PCO2, and SpO2). APGAR score was not statistically different between EP and NE at both 1 and 5 minutes. APGAR score showed no significant differ-ence indicating both drugs showed stable effect on maternal circulation to similar extant, which could explain the low rates of maternal adverse effect and similar rate between both groups. Ripolles, et al. report that hypotension following lumbar anesthesia can produce bradycardia in infants, as well as the chest tightness, dyspnea, nausea, and vomiting characteristic with puerpera, and hypoxia in the respiratory center [27]. In Fan, et al. study there was no difference in Apgar scores (1 and 5 minutes) and umbilical arterial blood gas analysis, PO2, and PCO2 between the two groups [18]. Chen, et al. examined three different doses of NE (5, 10, and 15 ��g/kg/h) that was given as infusion to pregnant women undergone CS and compare them to normal saline infusion (serve as control). The proportion of hypotension participants was significantly reduced in the NE groups (37.9%, 20%, and 25%, respectively) compared to that in the control group (86.7%). No significant difference in pH (7.33, 7.33, and 7.33 respectively), no significant differences in PO2 (24.3, 24.7, and 24.3 respectively), no significant differences in PCO2 (48.3, 50.2, and 48.3 respectively), no significant differences in HCO3 (20.2, 19.8, 21, and 19.6 respectively) [8]. This finding in agreement of our results in which NE stabilized neonatal blood gases and prevent acidosis. In Wang, et al. study, no significant difference in APGAR score, neonatal PO2, and PCO2. While pH (7.31 vs. 7.32), and HCO3 (24.1 vs. 22.2) was significantly different in EP compared to NE respectively [16]. In Huang, et al., they reported that APGAR score at birth (1 minute) was significantly lower in NE compared to EP (8.78 ± 0.32 vs. 8.86 ± 0.33), but later after 5 minutes no significant difference was noted (8.98 ± 0.34 vs. 9.05 ± 0.35) [22].
PCO2 and PO2 showed no significant difference between both medications, while pH was significantly higher in NE compared to EP (7.48 ± 0.10 vs. 7.42 ± 0.07) and SpO2 was significantly lower in NE compared to EP (91.74 ± 1.02, vs. 92.28 ± 1.14) [22]. Loubert, et al. [14] have showed that NE can promote uterine vasoconstriction through placenta, reduce fetal blood supply, and aggravate the hypoxic and ischemic state after birth [28]. In Vallejo, et al. study, an open label randomized trial, they found no significant difference in neonatal outcomes (APGAR score, pH, PCO2, PO2, and HCO3) [24]. Oxytocin is the first uterotonic used to stop bleeding after birth. But it can cause temporary low blood pressure (a drop of about 28 mmHg for 5 minutes) and cause the heart rate and cardiac output to go up, which can make the hemodynamics unstable [29]. Several studies reported that SBP, MBP, and SVR decreased and CO and HR increased after oxytocin administration [8]. But the effect of NE administration seems to ameliorate the effect of oxytocin and stabilize the hemodynamic fluctuations of the women [8]. Which is consistent with the current study, NE has both beta- and alpha-adrenergic effects, which could lead to a higher heart rate and cardiac output than EP and a lower chance of bradycardia [30].
Local anesthetic solution can be added to limit the area of local anesthesia and make it last longer. This reduces the amount of anesthetic used during a cesarean section, making sure that the mother and baby are even safer and easing the central neurological symptoms after the operation [31].
Adverse reactions
In the present study nausea was significantly higher in EP compared to NE groups, while no significant difference in other maternal side effects was noticed. This is related to the lower efficacy of EP to prevent hypotension compared to NE, and the high incidence of nausea is systemic reaction to high hypotension rate in EP. It is widely established that a vasopressor should be titrated to maintain blood pressure at or close to baseline levels in order to reduce maternal symptoms (nausea and vomiting) and fetal acidosis [32], thus explain why NE showed lower incidence of nausea compared to EP. In Abd Elraziq, et al. no significant difference in the incidence of nausea and vomiting between NE and EP [17]. In Vallejo, et al. study, the rate of emesis was significantly higher in EP compared to NE (26.3, vs. 16.3%), while nausea, and was not statistically significant [24]. This higher rate of emesis is attributed to patients who received intrathecal preservative free morphine 0.2 mg and fentanyl 20 μg for postoperative analgesia, while in the present study IV tramadol was used [24]. In Fan, et al. study the incidence of nausea and vomiting was significantly lower in NE (OR: 0.28, 95% CI: 0.11–0.70, P=0.004) [18]. In Wang, et al. study the incidence of nausea and vomiting was significantly higher EP compared to NE (20% vs. 5.4%) while no significant difference in shivering (5.5% vs. 7.1%) [16,33,34].
Conclusion
NE is an effective and safe vasopressor in preventing postanesthetic hypotension in SA for CS devilries, with less hypotensive episodes, better hemodynamic maternal effect, low incidence of nausea, and better neonatal umbilical cord blood gases compared to EP.
Supplementary Materials
The following supporting information can be downloaded at: https://doi.org/10.5281/zenodo.8025770.
Author Contributions
Conceptualization, SH, AI, and TA; methodology, SH, AI, HF, and TA; software, HF; validation, SH, AI, HF, and TA.; formal analysis, HF; investigation, SH, AI, and TA.; resources, SH, AI, HF, and TA.; data curation, SH, and HF; writing—original draft preparation, SH, and HF; writing—review and editing, SH, AI, HF, and TA; visualization, SH, and HF; supervision, AI, and TA; project administration, SH. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Iraqi Ministry of Health/ Environmental health office of Thi-Qar Training & development center (protocol code 270 and date of approval was 31/5/2021).
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Zenodo: Hayder Adnan Fawzi. (2023). Data of the Study [Data set]. Zenodo. https://doi.org/10.5281/zenodo.8025744
Data are available under the terms of the Creative Commons Attribution 4.0 International li-cense (CC-BY 4.0).
This is an open access database distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction, provided the original work is properly cited.
Conflicts of Interest
The authors declare no conflict of interest.
Authors Contribution
(A) Study Design · (B) Data Collection · (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Brill S, Gurman GM, Fisher A. A history of neuraxial administration of local analgesics and opioids. Eur J Anaesthesiol. 2003;20(9):682-689.
- Butterworth F. Morgan, Mikhail’s. Clinical Anesthesiology 5e. Appleton & Lange; 2013.
- Mercier FJ, Augè M, Hoffmann C, et al. Maternal hypotension during spinal anesthesia for caesarean delivery. Minerva Anestesiol. 2013;79(1):62-73.
- Fitzgerald JP, Fedoruk KA, Jadin SM, et al. Prevention of hypotension after spinal anaesthesia for caesarean section: A systematic review and network meta‐analysis of randomised controlled trials. Anaesthesia. 2020;75(1):109-121.
- Kee WD. The use of vasopressors during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2017;30(3):319-325.
- Kee WD, Lee A, Khaw KS, et al. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: The effects on fetal acid-base status and hemodynamic control. Anesthesia & Analgesia. 2008;107(4):1295-1302.
- Ngan Kee WD. A random-allocation graded dose–response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology. 2017;127(6):934-941.
- Chen D, Qi X, Huang X, et al. Efficacy and safety of different norepinephrine regimens for prevention of spinal hypotension in cesarean section: A randomized trial. Biomed Res Int. 2018;2018.
- Elnabtity AM, Selim MF. Norepinephrine vs. ephedrine to maintain arterial blood pressure during spinal anesthesia for cesarean delivery: A prospective double-blinded trial. Anesth Essays Res. 2018;12(1):92.
- De Diego Pdel R. Ephedrine vs. phenylephrine by intravenous bolus and continuous infusion to prevent hypotension secondary to spinal anesthesia during cesarean section: a randomized comparative trial. Rev Esp Anestesiol Reanim. 2011;58(7):412-416.
- Sharkey AM, Siddiqui N, Downey K, et al. Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat spinal-induced hypotension in cesarean deliveries: Randomized controlled trial. Anesth Analg. 2019;129(5):1312-1318.
- Kulkarni S, Gurudatt CL, Prakash D, et al. Effect of spinal flexion and extension in the lateral decubitus position on the unilaterality of spinal anesthesia using hyperbaric bupivacaine. J Anaesthesiol Clin Pharmacol. 2018;34(4):524.
- Hayder Adnan Fawzi. Ethical approval of (ID#: 270/31/5/2021; number: 0431570). Zenodo.2023.
- Ngan Kee WD, Lee SW, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122(4):736-745.
- Hassani V, Movaseghi G, Safaeeyan R, et al. Comparison of ephedrine vs. norepinephrine in treating anesthesia-induced hypotension in hypertensive patients: Randomized double-blinded study. Anesth Pain Med. 2018;8(4).
- Wang X, Mao M, Liu S, et al. A comparative study of bolus norepinephrine, phenylephrine, and ephedrine for the treatment of maternal hypotension in parturients with preeclampsia during cesarean delivery under spinal anesthesia. Med Sci Monit. 2019;25:1093.
- Abd Elraziq BA, Ali SF, Abotaleb UI, et al. Norepinephrine vs. Ephedrine in Prevention of Hypotension after Spinal Anesthesia. Al-Azhar Int Med J. 2020;1(10):37-42.
- Fan QQ, Wang YH, Fu JW, et al. Comparison of two vasopressor protocols for preventing hypotension post-spinal anesthesia during cesarean section: A randomized controlled trial. Chin Med J. 2021;134(07):792-799.
- Elhadidy S, Rafea M, Fawzy S, et al. Dynamic left intraventricular obstruction in patients with septic shock: Pathogenetic role and prognostic implications. Res Opinion Anaesth Intensive Care. 2019;6(4):424-428.
- Vallée F, Passouant O, Le Gall A, et al. Norepinephrine reduces arterial compliance less than phenylephrine when treating general anesthesia‐induced arterial hypotension. Acta Anaesthesiol Scand. 2017;61(6):590-600.
- Atashkhoie S, Pourfathi H, Naghipour B, et al. The effect of prophylactic infusion of combined ephedrin and phenylephrine on maternal hemodynamic after spinal anesthesia for cesarean section: A randomized clinical trial. Iran J Med Sci. 2018;43(1):70.
- Huang LX, Huang SN, Wu ZW. Effects of ephedrine, phenylephrine and norepinephrine prevent hypotension after spinal anesthesia in cesarean section and comparison of effects on newborns. J Hainan Med Univ. 2019;25(3):65-68.
- Mostafa M, Hasanin A, Mostafa M, et al. Hemodynamic effects of norepinephrine versus phenylephrine infusion for prophylaxis against spinal anesthesia-induced hypotension in the elderly population undergoing hip fracture surgery: A randomized controlled trial. Korean J Anesthesiol. 2021;74(4):308-316.
- Vallejo MC, Attaallah AF, Elzamzamy OM, et al. An open-label randomized controlled clinical trial for comparison of continuous phenylephrine vs. norepinephrine infusion in prevention of spinal hypotension during cesarean delivery. Int J Obstet Anesth. 2017;29:18-25.
- Ali HG, Abd ELatief N. Comparison of prophylactic phenylephrine infusion vs. intravenous ondansetron on hypotension during spinal anesthesia for cesarean section. Anesth Essays Res. 2022;16(2):226.
- Ngan Kee WD, Khaw KS, Tam YH, et al. Performance of a closed-loop feedback computer-controlled infusion system for maintaining blood pressure during spinal anaesthesia for caesarean section: A randomized controlled comparison of norepinephrine versus phenylephrine. J Clin Monit Comput. 2017;31:617-623.
- Melchor JR, Espinosa Á, Hurtado EM, et al. Colloids versus crystalloids in the prevention of hypotension induced by spinal anesthesia in elective cesarean section. A systematic review and meta-analysis. Minerva Anestesiol. 2015;81(9):1019-1030.
- Loubert C, Gagnon PO, Fernando R. Minimum effective fluid volume of colloid to prevent hypotension during caesarean section under spinal anesthesia using a prophylactic phenylephrine infusion: An up-down sequential allocation study. J Clin Anesth. 2017;36:194-200.
- Tantry TP, Karanth H, Anniyappa S, et al. Intravenous oxytocin regimens in patients undergoing cesarean delivery: A systematic review and network meta-analysis of cluster-based groups. J Anesth. 2023;37(2):278-293.
- Goel K, Luthra N, Goyal N, et al. Comparison of norepinephrine and phenylephrine infusions for maintenance of haemodynamics following subarachnoid block in lower segment caeserean section. Indian J Anaesth. 2021;65(8):600.
- McDonnell NJ, Paech MJ, Muchatuta NA, et al. A randomised double‐blind trial of phenylephrine and metaraminol infusions for prevention of hypotension during spinal and combined spinal–epidural anaesthesia for elective caesarean section. Anaesthesia. 2017;72(5):609-617.
- Carvalho B, Dyer RA. Norepinephrine for spinal hypotension during cesarean delivery: Another paradigm shift?. Anesthesiology. 2015;122(4):728-30.
- Hayder Adnan Fawzi. Supplemental tables. Zenodo. 2023.
- Hayder Adnan Fawzi.Data of the study [Data set]. Zenodo. 2023.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Sami R. Hasan1,2, Afraa Ibrahim1, Talib Razq M3 and Hayder A. Fawzi4*2Department of Anesthesia, College Health and Medical Technology, Sawa University, Samawah, Iraq
3Department of Anesthesia, College of Medicine, University of DhiQar, Dhi Qar Governorate, Iraq
4Department of Pharmacy, College of Pharmacy, Uruk University, Baghdad, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.