Research - (2023) Volume 18, Issue 4
Effect of anti-mullerian hormone in patients with polycystic ovarian syndrome on pregnancy rate in ICSI cycle
Ahmed Mohamed Abd El-Hay*, Mohammed Salah Elsayed Elsokkary and Bassem Aly IslamReceived: 03-Sep-2021, Manuscript No. gpmp-21-41070; Editor assigned: 04-Sep-2021, Pre QC No. P-41070; Reviewed: 21-Sep-2021, QC No. Q-41070; Revised: 10-Aug-2022, Manuscript No. R-41070; Published: 29-Dec-2023
Abstract
Background: Polycystic ovarian syndrome (PCOS) is the most frequent cause of anovulatory infertility and hyperandrogenism in young women. Women with this syndrome are characterized by an excessive number of small antral follicles (2–3 fold that of normal ovaries). Anti-Müllerian hormone (AMH), a member of the transforming growth factor-beta superfamily, it is mainly secreted in human ovary by the granulosa cells of ovarian early developing follicles. AMH is secreted inside preantral and early antral follicles <4 mm in diameter in granulosa cells. Its secretion decreases when the antral follicles begin to grow and stops when the follicles are larger 8 mm in diameter, or when atresia occurs. AMH levels are shown to be age-dependent, it is rarely detectable in new born baby girls and it reaches peaks after puberty and steadily declines with age until menopause when serum concentration becomes undetectable.
Objective: To evaluate the AMH in prediction of clinically pregnancy rate in patients with polycystic ovarian syndrome (PCOS) undergoing ICSI cycle.
Patients and Methods: The participants of this study were 140 PCOS women who were candidates for ICSI aged 20-40 years old with primary infertility for at least for 2 years and normal uterine cavity. Women with known metabolic disorders, pelvic pathologies as hydro-salpinges, myoma, stage IV endometriosis, history of myomectomy, history of ovarian hyper stimulation, previous ICSI cycles and/or cancelled cycle were excluded from the study. All participants underwent full history taking, physical examination, basic infertility tests and TVUS. Antagonist protocol was used for stimulation of ovulation. Pregnancy test divided the studied patients into two groups, 58% negative and 41.4% positive.
Results: There was statistically significant negative correlation between AMH level and age of the patients and total dose of ovulation induction. Receiver operating characteristic curve (ROC) for AMH level showed that the best cut off point was found > 3.3 ng/ml with sensitivity of 70.69%, specificity of 59.76% and area under curve of 0.672. Univariate logistic regression analysis showed that the pregnancy results was associated with AMH >3.3ng/ml, oocyte no >17, E2 >2746 pg/ml, embryo no >7, M2 >5, post maturation >2, A grade >2 and C grade >3. Also the multivariate logistic regression showed that the most important factors associated with positive pregnancy was found M 2 >5 with p-value <0.001 and OR (95.0% CI) of 9.322 (3.142-27.653) followed by AMH >3.3 with p-value=0.020 and OR (95% CI) of 2.964 (1.190-7.378).
Conclusion: From the results of the current study we can conclude that AMH had an important value in prediction of clinical pregnancy rate in patients with polycystic ovarian syndrome (PCOS) undergoing ICSI cycle. AMH level, oocytes number, E2 level, embryo number, M2 number, post maturation and grade A and C ICSI trial were higher in cases with positive pregnancy than negative pregnancy. AMH >3.3ng/ml, oocyte no >17, E2 >2746 pg/ml, embryo no >7, M2 >5, post maturation >2, A grade >2 and C grade >3 were associated with better clinical pregnancy rates. Poor AMH level led to higher doses of induction of ovulation
Keywords
Anti-mullerian hormone; Polycystic ovarian syndrome
Introduction
PCOS is the most frequent cause of anovulatory infertility and hyperandrogenism in young women. Women with this syndrome are characterized by an excessive number of small antral follicles (2–3 fold that of normal ovaries) [1].
Anti-Müllerian hormone (AMH), a member of the transforming growth factor-beta superfamily, it is mainly secreted in human ovary by the granulosa cells of ovarian early developing follicles, AMH is secreted inside preantral and early antral follicles <4 mm in diameter in granulosa cells. Its secretion decreases when the antral follicles begin to grow and stops when the follicles are larger 8 mm in diameter, or when atresia occurs. AMH levels are shown to be age-dependent, it is rarely detectable in newborn baby girls and it reaches peaks after puberty and steadily declines with age until menopause when serum concentration becomes undetectable [2].
The established predictors of reproductive potential during infertility treatment are maternal age, early follicular phase FSH concentrations, and less popularly, serum inhibin B concentration. None of these parameters is a particularly reliable predictor of the number or quality of oocytes remaining within the ovary, or the likelihood of pregnancy from infertility treatment. Recently, interest in the use of anti-müllerian hormone (AMH) and AFC to predict patient response to ovarian stimulation has been intense [3].
AMH levels in women with PCOS are two- to three-fold higher than those obtained in patients without PCOS [4].
Recently, anti-Müllerian hormone (AMH) has been considered a diagnostic or even prognostic marker of PCOS. In a recent study stated that the concentration of serum AMH might be related to the severity of PCOS and correlate with its clinical diagnostic hallmarks (i.e. hyperandrogenism, oligo/anovulation and polycystic ovary morphology [PCOM]). Pregnancy rates are likely to decrease with the exacerbation of PCOS. Although some studies have suggested a reverse relationship between the AMH concentration and pregnancy rate, some others have found a positive relationship between the AMH concentration, embryo quality and clinical pregnancy rates [5].
They also discover an increase in AMH levels in women with PCOS in which androgens may have a role in AMH regulation. Moreover, AMH levels were specifically studied to predict good quality embryos cryopreservation. It was evident that low AMH levels was statistically significantly associated with lower chances of blastocysts cryopreservation compared to high AMH levels in all women age [5].
Aim of the work
This study aims to evaluate the AMH in prediction of clinically pregnancy rate in patients with polycystic ovarian syndrome (PCOS) on pregnancy rate in ICSI cycle.
Patients and Methods
Type of study
Follow up Cohort study that was conducted on cases candidate for ICSI to evaluate the role of AMH in patients with PCOS on pregnancy rate in ICSI cycle.
Sample Size
Sample size was calculated using NCSS PASS 11.0 program and based on a study carried out by Xi et al., [1] and Chen et al., [4]. The sample size is calculated on two stages. Stage I : to measure the pregnancy rate in PCOS patients, based on the expected pregnancy rate among PCOS patients undergoing ICSI = 70%, assuming that the pregnancy rate is 70+5% at confidence level 95%, a sample size of 156% patients with PCOS is sufficient to achieve the study objectives. Stage II: to measure the relation between AMH and pregnancy rate. Group sample sizes of 70 in group one and 70 in group two achieve 80% power to detect a difference between the group proportions of -0.2300. The proportion in group one (High AMH) is assumed to be 0.5000 under the null hypothesis and 0.2700 under the alternative hypothesis. The proportion in group two (low AMH) is 0.5000. The test statistic used is the two-sided Z test with pooled variance. The significance level of the test was targeted at 0.0500. The significance level actually achieved by this design is 0.0515.
Study setting
Ain Shams University Hospital (Obstetrics & Gynecology Department).
Study period
6 months.
Study population
Patients
Inclusion criteria: Age (20 to 40 years). Females with primary infertility for at least for 2 years. Female with secondary infertility. Normal uterine cavity assessed on ultrasound, hysterosalpingogram or hysteroscopy were included. Patients with PCOS.
Exclusion criteria: Females with known metabolic disorders (Diabetes, Hypertension, Metabolic Syndrome). Pelvic pathologies (hydrosalpinges, myoma etc.). Presence of stage IV (severe) endometriosis. Past history of myomectomy. All the cases that developed hyper stimulation were not included irrespective of the fact that cycle was canceled or not. Patients who had done previous ICSI cycles. Patients with cancelled cycle.
N.B: PCOS patients diagnosed according to Rotterdam 2003 Criteria for PCOS (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group; 2004).
Two of the following three criteria are required:
- Oligo/anovulation
- Hyperandrogenism: Clinical (Hirsutism or less commonly male pattern alopecia) or Biochemical (raised FAI or free testosterone).
- Polycystic ovaries on ultrasound: Polycystic ovaries on ultrasound are diagnosed when more than 10 follicles encircling an echo-dense stroma measuring 2-8 mm in diameter contained in an ovary sizing more than 10 cc in volume.
Methods (study tools and procedures)
For every case, the following were done:
History & complete physical examination including:
- Age
- Measurement of BMI
- Past medical History
- Past Surgical History (including previous ICSI)
- Family history
- Husband history
- Serum Anti-Mullerian Hormone (AMH) Level at (day-3 before initiation of ovulation induction).
- FSH, LH and TSH
- Prolactin level
- Complete blood count (CBC)
- The Prothrombin Time (PT) and Partial Thromboplastin Time (PTT)
- HIV
- Hepatitis markers (for B & C)
- Random Blood Sugar (RBS)
- Semen analysis for husband
Radiological investigations:
- Antral follicle count done on the second day of the menstrual cycle by transvaginal scan (TVS).
- On the basis of beta-human chorionic gonadotropin (β-hCG) done 2 weeks after egg collection and TVS performed after another 2 weeks after identification of clinical pregnancy from pre-clinical abortion.
- On the basis of beta-human chorionic gonadotropin (β-hCG) and TVS, results were categorized into non-pregnant with β-hCG <25 mIU/ml and preclinical abortion β-hCG >25 mIU/ml with no fetal cardiac activity on TVS.
- Clinical pregnancy was identified by the existence of a gestational sac with cardiac movement perceived by TVS.
Study Procedures: We use the antagonist treatment protocol of females from controlled ovarian stimulation, ovulation induction (OI), oocyte pick up and embryo transfer.
Pregnancy rates: Effect of AMH on clinical pregnancy rate in PCOS patients.
Ethical Considerations: An informed consent was obtained from each patient before enrollment in the study, and the clinical examinations monitored principles of Declaration of Helsinki.
Statistical analysis: Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when parametric and median, inter-quartile range (IQR) when data found non-parametric. Also qualitative variables were presented as number and percentages. The Comparison between groups with qualitative data was done by using Chi-square test. The comparison between two groups with quantitative data and parametric distribution were done by using Independent t-test. While data with non-parametric distribution were done by using Mann-Whitney test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. Receiver operating characteristic curve (ROC) was used in the quantitative form to determine sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and Area under curve (AUC) of AMH between Negative and Positive pregnancy. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P >0.05: Non-significant. P <0.05: Significant. P <0.01: Highly significant.
Results
Tab.1-9. shows that there was no statistically significant difference found between the two studied groups regarding demographic and characteristics of the studied patients.
| Variables | No. = 140 | |
|---|---|---|
| AGE | Mean ± SD | 30.64 ± 5.65 |
| Range | 20-40 | |
| Body Mass Index (BMI) | Mean ± SD | 27.48 ± 3.23 |
| Range | 18.6-34.9 | |
| Duration of infertility | Median (IQR) | 6 (4-8) |
| Range | 1-20 | |
| Infertility type | Primary | 103 (73.6%) |
| Secondary | 37 (26.4%) | |
Tab. 1. Demographic and characteristics of the studied patients.
| Variables | No. | % | |
|---|---|---|---|
| Rhythm of menstrual cycle | Regular | 12 | 8.6% |
| Oligo-manrrhea | 128 | 91.4% | |
| History | Free | 135 | 96.4% |
| Ovarian cyst | 4 | 2.9% | |
| Hypothyroid | 1 | 0.7% | |
| HSG | Normal | 136 | 97.1% |
| Tubal block | 3 | 2.1% | |
| Filling defect | 1 | 0.7% | |
| Semen analysis | Normal | 84 | 60.0% |
| Oligo-Astheno-Teratozoospermia (OAT) | 56 | 40.0% | |
Tab. 2. A table showing the history and Hysterosalpingogram (HSG) results of the studied patients.
| Variables | No. = 140 | |
|---|---|---|
| FSH | Mean ± SD | 5.55 ± 2.13 |
| Range | 1.2-10.6 | |
| LH | Mean ± SD | 6.83 ± 2.91 |
| Range | 1.2-18 | |
| TSH | Median (IQR) | 2.1 (1.4-2.8) |
| Range | 0.09-18.2 | |
| Prolactin (PRL) | Median (IQR) | 13.75 (10-18) |
| Range | 2.7-40.8 | |
Tab. 3. Laboratory data of the studied patients.
| Variables | No. = 140 | |
|---|---|---|
| Starting dose | 75 | 20 (14.3%) |
| 150 | 120 (85.7%) | |
| Duration of HMG | Mean ± SD | 8.99 ± 0.92 |
| Range | 8-12 | |
| Total dose | Mean ± SD | 1685.36 ± 284.48 |
| Range | 900-3000 | |
| E2 | Median (IQR) | 2782 (1993-4008.5) |
| Range | 1041-8560 | |
Tab. 4. A table showing the ST dose, duration, total dose of HMG and E2 of the studied patients.
| Variables | No. = 140 | |
|---|---|---|
| Endometrial thickness | Mean ± SD | 11.88 ± 1.92 |
| Range | 8-18 | |
| Endometrial shape | Triple | 99 (70.7%) |
| Homogenous | 41 (29.3%) | |
Tab. 5. Endometrial thickness and endometrial shape among the studied patients.
| Variables | Median (IQR) | Range |
|---|---|---|
| AMH | 3.55 (2.5-5.1) | 1-16 |
| Oocyte number (ocyte No.) | 15.91 ± 5.49 | 7-32 |
| Embryo number (embryo No.) | 6 (4-8.5) | 2-17 |
| G.V number (G.V No.) | 3 (2-4) | 0-13 |
| M1 | 4 (2-6) | 0-12 |
| M2 | 5 (3-7) | 0-14 |
| Atretic | 1 (0-2) | 0-8 |
| Post Maturation | 1 (1-2) | 0-7 |
Tab. 6. AMH level, oocyte, embryo and GV numbers, M1, M2, atretic and post maturation results of the studied patients.
| Grades | Median (IQR) | Range |
|---|---|---|
| A Grade | 3 (2-5) | 0-10 |
| B Grade | 2 (1-3) | 0-14 |
| C Grade | 1 (0-2) | 0-7 |
| ICSI Trial | 1 (1-1) | 0-3 |
Tab. 7. A, B, and C grades and ICSI trial among the studied patients.
| Pregnancy test | No. | % |
|---|---|---|
| Negative | 82 | 58.6% |
| Positive | 58 | 41.4% |
| Total | 140 | 100.0% |
Tab. 8. Results of pregnancy test among the studied patients.
| Variables | Negative Pregnancy | Pregnancy | Test value | P-value | Sig. | |
|---|---|---|---|---|---|---|
| No. = 82 | No. = 58 | |||||
| Age | Mean ± SD | 31.25 ± 5.97 | 29.78 ± 5.07 | 1.524• | 0.130 | NS |
| Range | 20-40 | 20-40 | ||||
| BMI | Mean ± SD | 27.72 ± 3.05 | 27.14 ± 3.46 | 1.048• | 0.296 | NS |
| Range | 18.6-34.7 | 20.17-34.9 | ||||
| Infertility Type | Primary | 58 (70.7%) | 45 (77.6%) | 0.821* | 0.365 | NS |
| Secondary | 24 (29.3%) | 13 (22.4%) | ||||
| Duration of infertility |
Median (IQR) | 6 (4-8) | 6 (4-8) | -0.043ǂ | 0.966 | NS |
| Range | 2-20 | 1-17 | ||||
*:Chi-square test; •: Independent t-test; ‡: Mann Whitney test
Tab. 9. Comparison between cases with negative pregnancy test and positive pregnancy test regarding demographic data and characteristics of the studied patients.
Tab. 10. shows that there was no statistically significant difference found between the two studied groups regarding history and hysterosalpingogram results.
| Variables | Negative Pregnancy | Pregnancy | Test value* | P-value | Sig. | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| Rhythm of menstrual cycle | Regular | 7 | 8.5% | 5 | 8.6% | 0.000 | 0.986 | NS |
| Oligo-manrrhea | 75 | 91.5% | 53 | 91.4% | ||||
| History | Free | 80 | 97.6% | 55 | 94.8% | 2.663 | 0.447 | NS |
| Ovarian cyst | 1 | 1.2% | 3 | 5.2% | ||||
| Hypothyroid | 1 | 1.2% | 0 | 0.0% | ||||
| HSG | Normal | 79 | 96.3% | 57 | 98.3% | 3.549 | 0.170 | NS |
| Tubal block | 3 | 3.7% | 0 | 0.0% | ||||
| Filling defect | 0 | 0.0% | 1 | 1.7% | ||||
| Semen analysis | Normal | 50 | 61.0% | 34 | 58.6% | 0.078 | 0.779 | NS |
| Oligo-Astheno-Teratozoospermia (OAT) | 32 | 39.0% | 24 | 41.4% | ||||
*:Chi-square test
Tab. 10. Comparison between cases with negative pregnancy test and positive pregnancy test regarding history
Tab. 11. shows that there was no statistically significant difference found between the two studied groups regarding laboratory data.
| Variables | Negative Pregnancy | Pregnancy | Test value | P-value | Sig. | |
|---|---|---|---|---|---|---|
| No. = 82 | No. = 58 | |||||
| FSH | Mean ± SD | 5.81 ± 2.19 | 5.18 ± 2.01 | 1.723• | 0.087 | NS |
| Range | 1.2-10.6 | 1.5-10.6 | ||||
| LH | Mean ± SD | 6.87 ± 2.80 | 6.79 ± 3.08 | 0.156• | 0.877 | NS |
| Range | 1.2-18 | 1.3-14.3 | ||||
| TSH | Median (IQR) | 2.1 (1.4-2.8) | 2.1 (1.5-3.1) | -0.800ǂ | 0.424 | NS |
| Range | 0.09-18.2 | 0.5-13.2 | ||||
| PRL | Median (IQR) | 13.7 (10.5-17.3) | 14.95 (9.2-19) | -0.027ǂ | 0.978 | NS |
| Range | 2.7-40 | 3.1-40.8 | ||||
*:Chi-square test
Tab. 11. Comparison between cases with negative pregnancy test and positive pregnancy test regarding laboratory data
Tab. 12. shows that there was statistically significant difference found between the two studied groups regarding AMH level which was higher in cases with positive pregnancy than negative pregnancy with p-value=0.001 while no statistically significant difference found between both groups regarding starting dose, duration of HMG and total dose.
| Variables | Negative Pregnancy | Pregnancy | Test value | P-value | Sig. | |
|---|---|---|---|---|---|---|
| No. = 82 | No. = 58 | |||||
| AMH | Median (IQR) | 2.95 (2.1-4.2) | 4.05 (3.1-5.7) | -3.457ǂ | 0.001 | HS |
| Range | 1-16 | 2.1-8.5 | ||||
| Starting dose | 75 | 9 (11.0%) | 11 (19.0%) | 1.771* | 0.183 | NS |
| 150 | 73 (89.0%) | 47 (81.0%) | ||||
| Duration of HMG |
Mean ± SD | 8.95 ± 0.98 | 9.05 ± 0.83 | -0.637• | 0.525 | NS |
| Range | 8-12 | 8-11 | ||||
| Total Dose | Mean ± SD | 1697.87 ± 307.62 | 1667.67 ± 249.65 | 0.617• | 0.538 | NS |
| Range | 900-3000 | 1125-2400 | ||||
•: Independent t-test; ‡: Mann Whitney test
Tab. 12. Comparison between cases with negative pregnancy test and positive pregnancy test regarding AMH, starting dose, duration of HMG and total dose.
Tab. 13. shows that there was statistically significant difference found between the two studied groups regarding oocytes number, E2, embryo number, M2 and post maturation while no statistically significant difference found between the two groups regarding the other parameters.
| Variables | Negative Pregnancy | Pregnancy | Test value | P-value | Sig. | |
|---|---|---|---|---|---|---|
| No. = 82 | No. = 58 | |||||
| Endometrial thickness | Mean ± SD | 11.74 ± 1.96 | 12.07 ± 1.86 | -0.988• | 0.325 | NS |
| Range | 8-17 | 9-18 | ||||
| Endometrial shape |
Triple | 53 (64.6%) | 46 (79.3%) | 3.533* | 0.060 | NS |
| Homogenous | 29 (35.4%) | 12 (20.7%) | ||||
| Oocy No. | Mean ± SD | 14.48 ± 4.64 | 17.93 ± 5.98 | -3.846• | 0.000 | HS |
| Range | 7-27 | 9-32 | ||||
| E2 | Median (IQR) | 2356.5 (1750-3140) | 3213 (2327-4762) | -3.731ǂ | 0.000 | HS |
| Range | 1041-6501 | 1099-8560 | ||||
| Embryo No. | Median (IQR) | 5 (4-7) | 8 (5-11) | -4.002ǂ | 0.000 | HS |
| Range | 2-16 | 3-17 | ||||
| G.V No. | Median (IQR) | 3 (2-4) | 3 (1-5) | -0.467ǂ | 0.641 | NS |
| Range | 1-11 | 0-13 | ||||
| M1 | Median (IQR) | 4 (2-6) | 4.5 (3-7) | -1.052ǂ | 0.293 | NS |
| Range | 0-9 | 0-12 | ||||
| M2 | Median (IQR) | 4 (3-5) | 6 (5-8) | -6.272ǂ | 0.000 | HS |
| Range | 0-13 | 3-14 | ||||
| Atretic | Median (IQR) | 1 (0-2) | 0 (0-2) | -1.647ǂ | 0.100 | NS |
| Range | 0-8 | 0-5 | ||||
| Post maturation | Median (IQR) | 1 (0-2) | 2 (1-3) | -2.110ǂ | 0.035 | S |
| Range | 0-6 | 0-7 | ||||
*:Chi-square test; •: Independent t-test; ‡: Mann Whitney test
Tab. 13. Comparison between cases with negative pregnancy test and positive pregnancy test regarding endometrial thickness, endometrial shape, number of oocytes, embryos and GV, M1, M2, atretic and post maturation.
Tab. 14. shows that there was statistically significant difference found between the two studied groups regarding A grade and C grade while no statistically significant difference found between the two groups regarding B grade and ICSI trial.
| Variables | Negative Pregnancy | Pregnancy | Test value ǂ | P-value | Sig. | |
|---|---|---|---|---|---|---|
| No. = 82 | No. = 58 | |||||
| A Grade | Median (IQR) | 2.5 (1-4) | 4 (3-5) | -3.768 | 0.000 | HS |
| Range | 0-8 | 1-10 | ||||
| B Grade | Median (IQR) | 2 (1-3) | 2 (1-3) | -1.258 | 0.208 | NS |
| Range | 0-14 | 0-7 | ||||
| C Grade | Median (IQR) | 0 (0-2) | 1 (0-3) | -2.054 | 0.040 | S |
| Range | 0-5 | 0-7 | ||||
| ICSI Trial | Median (IQR) | 1 (1-1) | 1 (1-1) | -1.570 | 0.116 | NS |
| Range | 0-3 | 0-2 | ||||
‡: Mann Whitney test
Tab. 14. Comparison between cases with negative pregnancy test and positive pregnancy test regarding grades and ICSI trial.
Tab. 15. shows that there was no statistically significant difference found between the two studied groups regarding type of transfer (Fresh vs. Frozen).
| Variables | Negative pregnancy | Positive pregnancy | Test value | P-value | Sig. | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Fresh | 48 | 58.5% | 28 | 48.3% | 1.441 | 0.230 | NS |
| Frozen | 34 | 41.5% | 30 | 51.7% | |||
Tab. 15. Comparison between cases with negative pregnancy test and positive pregnancy test regarding type of transfer (Fresh Vs Frozen).
The ROC curve shows that the best cut off point was found >3.3 with sensitivity of 70.69%, specificity of 59.76% and area under curve of 0.672 (Fig. 1. and Tab. 16.).
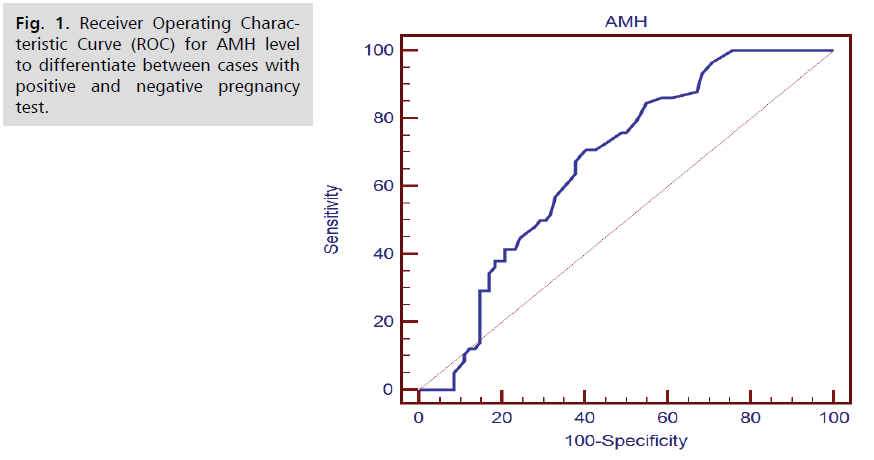
Fig. 1. Receiver Operating Characteristic Curve (ROC) for AMH level to differentiate between cases with positive and negative pregnancy test.
| Parameter | AUC | Cut of Point | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| AMH | 0.672 | >3.3 | 70.69 | 59.76 | 55.4 | 74.2 |
Tab. 16. AMH level to differentiate between cases with positive and negative pregnancy test.
Tab. 17. shows that there was statistically significant positive correlation found between AMH level and oocytes number, E2, embryo number, M1, M2, A grade and B grade and also negative correlation between AMH level and age of the patients and total dose while no statistically significant correlation found between AMH level and the other parameters.
| Variables | AMH | |
|---|---|---|
| R | P-value | |
| AGE | -0.361** | 0.000 |
| Body Mass Index (BMI) | -0.147 | 0.083 |
| Duration of infertility | 0.029 | 0.738 |
| FSH | -0.042 | 0.624 |
| LH | 0.133 | 0.117 |
| TSH | 0.016 | 0.849 |
| Prolactin (PRL) | -0.020 | 0.813 |
| Duration of HMG | -0.024 | 0.782 |
| Total Dose of HMG | -0.172* | 0.042 |
| Endometrial Thickness | 0.092 | 0.278 |
| Oocy No. | 0.419** | 0.000 |
| E2 | 0.446** | 0.000 |
| Emryo No. | 0.315** | 0.000 |
| G.V No. | 0.049 | 0.562 |
| M1 | 0.405** | 0.000 |
| M2 | 0.236** | 0.005 |
| Atretic | 0.105 | 0.220 |
| Post maturation | 0.140 | 0.099 |
| A Grade | 0.217* | 0.010 |
| B Grade | 0.277** | 0.001 |
| C Grade | 0.138 | 0.105 |
| ICSI Trial | -0.104 | 0.220 |
Tab. 17. Correlation of AMH level with the other studied parameters.
Tab. 18. shows that there was statistically significant relation found between infertility type and AMH level while no statistically significant relation found between AMH and rhythm of menstrual cycle, starting dose of HMG, and history.
| Variables | AMH | Test value | P-value | Sig. | ||
|---|---|---|---|---|---|---|
| Median (IQR) | Range | |||||
| Infertility Type | Primary | 3.9 (2.6-5.6) | 1-16 | -3.198ǂ | 0.001 | HS |
| Secondary | 2.8 (1.9-3.8) | 1.3-9.4 | ||||
| Rhythm of menstrual cycle | Regular | 3.45 (2.6-4.2) | 1-8.4 | -0.372ǂ | 0.710 | NS |
| Oligo-manrrhea | 3.6 (2.45-5.15) | 1.2-16 | ||||
| Starting dose | 75 | 4.8 (3.4-6) | 2.7-8.4 | -0.923ǂ | 0.356 | NS |
| 150 | 3.9 (2.9-5.7) | 2.1-8.5 | ||||
| History | Free | 3.5 (2.5-5.1) | 1-16 | 1.779 | 0.411 | NS |
| Ovarian cyst | 4.55 (3.35-5.35) | 2.8-5.5 | ||||
| Hypothyroid | 2.3 (2.3-2.3) | 2.3-2.3 | ||||
‡: Mann Whitney test; ‡‡: Kruskal Wallis test
Tab. 18. Relation of AMH level with the other parameters.
The previous univariate logistic regression analysis shows that the pregnancy results was associated with AMH >3.3, oocyte no >17, E2 >2746, embryo no >7, M2 >5, post maturation >2, A grade >2 and C grade >3. Also the multivariate logistic regression shows that the most important factors associated with positive pregnancy was found M 2 >5 with p-value <0.001 and OR (95.0% CI) of 9.322 (3.142-27.653) followed by AMH >3.3 with p-value=0.020 and OR (95% CI) of 2.964 (1.190–7.378) (Tab. 19.).
| Variables | Uni-variate | Multi-variate | ||||||
|---|---|---|---|---|---|---|---|---|
| P-value | Odds ratio (OR) | 95% C.I. for OR | P-value | Odds ratio (OR) | 95% C.I. for OR | |||
| Lower | Upper | Lower | Upper | |||||
| AMH >3.3 | 0.000 | 3.581 | 1.748 | 7.337 | 0.020 | 2.964 | 1.190 | 7.378 |
| Oocy No. >17 | 0.000 | 4.169 | 1.948 | 8.921 | 0.406 | 1.686 | 0.491 | 5.783 |
| E2 >2746 | 0.000 | 3.968 | 1.931 | 8.154 | 0.335 | 1.647 | 0.597 | 4.542 |
| Embryo No. >7 | 0.000 | 3.810 | 1.829 | 7.936 | 0.382 | 0.574 | 0.166 | 1.992 |
| M2 >5 | 0.000 | 9.970 | 4.504 | 22.071 | 0.000 | 9.322 | 3.142 | 27.653 |
| Post Maturation >2 | 0.012 | 2.794 | 1.252 | 6.234 | 0.508 | 0.648 | 0.179 | 2.341 |
| A Grade >2 | 0.002 | 3.143 | 1.498 | 6.594 | 0.866 | 1.095 | 0.381 | 3.149 |
| C Grade >3 | 0.004 | 4.900 | 1.654 | 14.519 | 0.712 | 1.324 | 0.299 | 5.875 |
Tab. 19. Uni-variate and multivariate logistic regression analysis for factors associated with positive pregnancy.
Discussion
This follow up cohort study was conducted at the department of obstetrics and gynecology, infertility outpatient clinic and Assisted Reproductive Technique Unit (ARTU) at Ain Shams University Maternity Hospital (ASUMH) in the period between April 2020 and April 2021.
This study aimed to evaluate the AMH value in prediction of cumulative clinical pregnancy rate in patients with polycystic ovarian syndrome (PCOS) undergoing ICSI cycle.
The participants of this study were 140 PCOS women who were candidates for ICSI aged 20-40 years old with primary infertility for at least for 2 years and normal uterine cavity.
Women with known metabolic disorders, pelvic pathologies as hydro-salpinges, myoma, stage IV endometriosis, history of myomectomy, history of ovarian hyper stimulation, previous ICSI cycles and/or cancelled cycle were excluded from the study.
All participants underwent full history taking, physical examination, basic infertility tests and TVUS. Antagonist protocol was used for stimulation of ovulation. Studied patients were divided into two groups, 58% negative and 41.4% positive pregnancy test.
Regarding demographic data and characteristics of the studied patients of current study, there was no statistically significant difference found between the two studied groups regarding basal demographic data as age, BMI and characteristics of the studied patients as infertility type, duration, rhythm of menstrual cycle, medical history, history of ovarian cysts, hystero-salpingo-gram results, semen analysis, hormonal profile (FSH, LH, TSH, PRL) and type of transfer (fresh vs. frozen).
However there was statistically significant negative correlation between AMH level and age of the patients and total dose of ovulation induction.
Tal et al., [5] aimed to assess the predictive value of serum AMH value in live birth outcomes in PCOS cases undergoing assisted reproductive technology. This was a retrospective cohort study of 184 PCOS women (Rotterdam criteria) who underwent their first fresh IVF/ICSI cycle. Women were divided into 3 groups according to the <25th (low), 25 to 75th (average), or >75th (high) percentile of serum AMH concentration. Cycle stimulation parameters and reproductive outcomes were compared between groups. They agreed with the current study and stated that there were no significant differences between study groups regarding the main baseline “age and BMI” and cycle characteristics. They also agreed with current study and stated that AMH levels decreased with increasing age of women in current study, with women in the low AMH group being significantly older compared to the average and high AMH groups. They disagreed with current study and stated that there was little variation in BMI between the different AMH level groups in this study that may be due to different number of obese women between the two studies. They agreed with current study and stated that there was an inverse relationship between AMH levels and gonadotropin dose, which was significant in the <25th percentile group (3316 ± 1288 IU) compared to the 25 to 75th percentile (2587 ± 1071 IU) and > 75th percentile (2252 ± 842 IU) groups.
Sahmay et al., [3] aimed to investigate the role of serum anti-Mullerian hormone (AMH), follicle-stimulating hormone (FSH) and antral follicle count (AFC) for the prediction of clinical pregnancy rates in women with polycystic ovary syndrome (PCOS) undergoing IVF treatment. A total of 150 consecutive women with PCOS who were admitted to Istanbul University Cerrahpasa School of Medicine, IVF Center of Reproductive Endocrinology and Infertility department from February 2010 to June 2012 were enrolled in this prospective cohort study. All women underwent gonadotropin-releasing hormone (GnRHa) agonist, leuprolide acetate 1 mg/d s.c. (Lucrin, Cedex, France) beginning on the 21st day of the previous cycle. They agreed with the current study and stated that there was no significant difference in terms of mean age, duration of infertility, BMI, AMH, LH, FSH, E2, TSH, AFC and the total number of oocytes between pregnant and non-pregnant women.
Rida [8] aimed to identify the role of Anti-Müllerian hormone (AMH) in prediction of outcome of intracytoplasmic sperm injection (ICSI) in patients with PCOS. A retrospective study was conducted in the period from January to March 2018. Eighty Sudanese women aged between 21 to 47 years old with PCOS underwent ICSI were recruited in this study. The selection of patients was done at Dr. Elsir Abu-Elhassan fertility center, Khartoum-Sudan. The Current study did not evaluate the relation between maternal age and ICSI outcomes They stated that the odds ratio (odds ratio >1) indicate there was a high chance to get success ICSI with women of age <35 years compared to women of age >35 years.
Zakaria et al., [7] aimed to assess role of serum AMH, FSH and AFC measurement as a prediction of pregnancy rates in IVF/intracytoplasmic sperm injection (ICSI) cycle. A prospective cross sectional study was conducted at Specialized Air Forced Hospital in the period between January 2018 and December 2018. The study included 100 unexplained infertility patients undergoing IVF/ICSI treatment cycles. They were 82 patients primary infertility and 18 patients secondary infertility. They agreed with the current study and stated that type of infertility showed insignificant difference between the two groups. The mean value of FSH, day 3 E2 levels did not have any significant difference between the two groups. They disagreed with the current study and stated that the menstrual history and gonadotrophins type showed significant difference. Age and duration of infertility were significantly higher in non-pregnant group than the pregnant one. This may be due to the different population inclusion criteria compared with current study.
In the current study, there was statistically significant difference found between the two studied groups regarding AMH level, oocytes number, E2, embryo number, M2, post maturation and grade A and C ICSI trial which was higher in cases with positive pregnancy than negative pregnancy with p-value = 0.001 while no statistically significant difference was found between both groups regarding starting dose, duration of HMG and total dose of ovulation induction. There was statistically significant positive correlation found between AMH level and oocytes number, E2, embryo number, M1, M2, A grade and B grade.
Chen et al., [4] evaluated associations of basal serum and follicular fluid (FF) anti Mullerian hormone (AMH) levels with in vitro fertilization (IVF) outcomes in polycystic ovary syndrome (PCOS) patients. This prospective study included 179 consecutive women undergoing IVF, including 59 with PCOS and non-PCOS controls. Thirty PCOS cases had long gonadotrophin-releasing hormone agonist (GnRH-a) and 29 had antagonist (GnRH-ant) protocols. Controls underwent conventional GnRH-a. Associations of basal serum and FF AMH levels with IVF outcomes were assessed. They agreed with current results and stated that AMH levels were correlated with AFC in PCOS patients (P <0.01). Peak E2 and AMH levels were independent predictors of oocyte number. They disagreed with the current results and stated that serum basal AMH levels are predictive of oocyte quantity, but not oocyte quality or IVF outcomes and that may be due to using of two protocols of ovulation induction. Serum AMH and outcomes were similar among protocols however, the current study used the antagonist protocol only.
Tal et al., [6] disagreed with the current study and stated that no differences were noted between groups in terms of maximal E2, oocytes retrieved and fertilization rate that may be due to different methodology comparing with the current study as they divided their population into three groups regarding AMH level. However, low serum AMH women had significantly greater live birth rates (p <0.05) and showed a trend towards greater clinical pregnancy rates compared to women in the average and high AMH groups (p=0.09).
They agreed with the current study and stated that from the low to high AMH ranges, the average number of oocytes retrieved increased from 12.6 ± 6.3, 13.4 ± 6.2, to 14.2 ± 8.0, respectively. The number of fertilizations was highest in the high AMH range as well at 9.5 ± 5.6. Approximately two embryos on average were transferred for each group per procedure. In the current study univariate logistic regression analysis showed that the pregnancy results was associated with AMH >3.3. Multivariate logistic regression showed that the most important factors associated with positive pregnancy was found AMH >3.3 with p-value =0.020 and OR (95% CI) of 2.964 (1.190-7.378).
Tal et al., [6] stated that there was significant increase in live birth rate (LBR) per embryo transfer in PCOS women in the low AMH group (65.2%) compared to the average AMH (46.7%) and high AMH (43.5%) groups. The odds ratio (OR) for LBR in the low AMH group compared to the other groups (average and high) was 2.29 (p=0.017) and remained significant after adjusting for age, BMI, day of transfer and number of embryos transferred.
Sahmay et al., [3] disagreed with the current study and stated that AMH was not predictive for clinical pregnancy rate in women with PCOS undergoing IVF treatment. Mean AMH values were not significantly different between pregnant and non-pregnant women. Although clinical pregnancy rate increased in parallel with the raise in AMH percentiles, this remained insignificant. This may be due to that they used the agonist protocol for induction.
Rida [8] disagreed with the current study and stated that there was no statistically significant difference in the levels of AMH between the groups of women who had got successful pregnancy compared to those who did not have. They stated that there was no significant difference (P >0.05) in AMH level and FSH, whereas there was a significant difference in LH and LH/FSH ration between the two groups. This may be due to the different sample sizes between the two studies.
Ramezanali et al., [5] aimed to evaluate IVF/intracytoplasmic sperm injection (ICSI) outcomes in different polycystic ovary syndrome (PCOS) phenotypes (A, B, C and D) compared with a control group and the predictive values of serum anti-Müllerian hormone (AMH) in PCOS phenotypes for main outcomes. This study evaluated 386 PCOS women and 350 patients with male factor infertility.
PCOS patients were categorized to four phenotype groups according to the Rotterdam criteria: (i) phenotype A: the coexistence of hyperandrogenism, chronic anovulation and polycystic ovaries (HA+AO+PCO); (ii) phenotype B: chronic anovulationand hyperandrogenism without the polycystic ovaries (AO+HA); (iii) phenotype C: hyperandrogenism and polycystic ovaries (HA+PCO); and (iv) phenotype D: polycystic ovaries coexisting with anovulatory cycles (AO+PCO). They disagreed with the current study and stated that the AMH concentration is related to PCO morphology but not predictive for clinical pregnancy rate and live birth rate. This may be due to that the current study did not classify PCOS cases into phenotypes.
Ramezanali et al., [5] stated that women with phenotypes A and C had significantly higher concentrations of AMH than those with phenotype B (P <0.001). Clinical pregnancy rate in the phenotype D group (53.3%) was higher than other groups (32.5%, 26.4% and 36.8%, respectively, in phenotypes A, B and C), but not to a significant level. Multivariable regression analysis, after adjusting for women’s age and body mass index, revealed that PCOS phenotypes A and B were associated with a decreased CPR compared with the control group (odds ratio [OR]: 0.46, confidence interval [CI]: 0.26-0.8, P=0.007 and OR: 0.34, CI: 0.18-0.62, P =0.001, respectively). It seems a combination of hyperandrogenism and chronic anovulation is associated with a negative impact on the CPR in those patients.
Zakaria et al., [7] stated that no test can predict pregnancy in high accuracy as it is multifactorial however, mean levels of FSH, E2, a day HCG injection and AFC showed significant difference between pregnant and non-pregnant women. They agreed with the current study and stated that the mean value of serum E2, the day of HCG injection, serum AMH day 3 and AFC were significantly higher in pregnant group than non-pregnant.
Tal et al., [6] aimed to assess whether antimullerian hormone (AMH) is a predictor of implantation and/or clinical pregnancy in women undergoing assisted reproductive technology. They stated that Antimullerian hormone has weak association with implantation and clinical pregnancy rates in assisted reproductive technology but may still have some clinical utility in counseling women undergoing fertility treatment regarding pregnancy rates, particularly those with diminished ovarian reserve.
In the current study, receiver operating characteristic curve (ROC) for AMH level was done to differentiate between cases with positive and negative pregnancy test. It showed that the best cut off point was found >3.3 ng/ml with sensitivity of 70.69%, specificity of 59.76% and area under curve of 0.672. Univariate logistic regression analysis showed that the pregnancy results was associated with AMH >3.3ng/ml, oocyte no >17, E2 >2746pg/ml, embryo no >7, M2 >5, post maturation >2, A grade >2 and C grade >3. Also the multivariate logistic regression showed that the most important factors associated with positive pregnancy was found M 2 >5 with p-value <0.001 and OR (95.0% CI) of 9.322 (3.142-27.653) followed by AMH >3.3 with p-value=0.020 and OR (95% CI) of 2.964 (1.190-7.378).
Tal et al., [6] disagreed with the current study and stated that Receiver-operating characteristic (ROC) curve analysis for AMH as a predictor of live birth revealed an area under the curve (AUC) of 0.543 (0.424-0.661, 95% confidence interval) indicating overall poor predictability for live birth. Although not statistically significant, the same inverse trend with AMH concentration was found for clinical pregnancy rate (CPR) (69.6, 53.2, 52.2%), implantation rate (IR) (47.1, 40.6, 35.3%), and multiple pregnancy rate (26.0, 21.7, 13.0%). Fertilization rate was highest in the low AMH range (70.4%), but there was not a uniform inverse relationship with this outcome. This may be due to the different number of the obese women between the two studies and the different methodology.
Sahmay et al., [3] disagreed with the current study and stated that the cut-off levels of AMH in the 25th and 75th percentiles were 4.23 and 8.66 ng/mL respectively. Mean FSH was 5.44 ± 3.83 and 4.78 ± 2.81 mIU/mL in pregnant and nonpregnant women, respectively (p =0.484). Cut-off levels of FSH in the 25th and 75th percentiles were 4.03 and 5.98 mIU/mL respectively. Mean AFC was 13.61 ± 5.32 and 12.25 ± 5.33 in pregnant and non-pregnant women, respectively (p =0.165). Cut-off levels of AFC in the 25th and 75th percentiles were 9 and 17 follicles, respectively. This may be due to that they used the agonist protocol for induction.
Zakaria et al., [7] disagreed with the current study and stated that the ability of AMH to predict good responders was with cut-off point >1.3 ng/ml with sensitivity, specificity, PPV, NPV and accuracy 85.8%, 92.0%, 83.8%, 93.0% and 89.0% respectively. This may be due to the different population inclusion criteria compared with the current study.
The OR for AMH as a predictor of implantation in women with unspecified ovarian reserve (n=1.591) was 1.83 (95% confidence interval [CI] 1.49-2.25), whereas the AUC was 0.591 (95% CI 0.563-0.618). The OR for AMH as a predictor of clinical pregnancy in these women (n=4.324) was 2.10 (95% CI 1.82-2.41), whereas the AUC was 0.634 (95% CI 0.618-0.650).
The predictive ability of AMH for pregnancy was greatest in women with DOR (n=615), with OR and AUC of 3.96 (95% CI 2.57-6.10) and 0.696 (95% CI 0.641-0.751), respectively. In contrast, AMH had no significant predictive ability in women with PCOS (n=414), with OR and AUC of 1.18 (95% CI 0.53-2.62) and 0.600 (95% CI 0.547-0.653), respectively. This may be due to the different number of the obese women between the two studies and the different methodology.
Conclusion
From the results of the current study we can conclude that AMH had an important value in prediction of clinical pregnancy rate in patients with polycystic ovarian syndrome (PCOS) undergoing ICSI cycle. AMH level, oocytes number, E2 level, embryo number, M2 number, post maturation and grade A and C ICSI trial were higher in cases with positive pregnancy than negative pregnancy. AMH >3.3ng/ml, oocyte no >17, E2 >2746 pg/ml, embryo no >7, M2 >5, post maturation >2, A grade >2 and C grade >3 were associated with better clinical pregnancy rates. Poor AMH level led to higher doses of induction of ovulation.
Authors Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) Funds Collection
References
- Xi W, Gong F, Lu G. Correlation of serum Anti-Müllerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle with assisted reproduction outcome in polycystic ovary syndrome patients. J Assist Reprod Genet. 2012;29(5):397-402.
- Lie Fong S, Visser JA, Welt CK, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-5.
- Sahmay S, Guralp O, Aydogan B, et al. Anti-Müllerian hormone and polycystic ovary syndrome: assessment of the clinical pregnancy rates in in vitro fertilization patients. Gynecol Endocrinol. 2013;29(5):440-3.
- Chen Y, Ye B, Yang X, et al. Predicting the outcome of different protocols of in vitro fertilization with anti-Muüllerian hormone levels in patients with polycystic ovary syndrome. J Int Med Res. 2017;45(3):1138-47.
- Ramezanali F, Ashrafi M, Hemat M, et al. Assisted reproductive outcomes in women with different polycystic ovary syndrome phenotypes: the predictive value of anti-Müllerian hormone. Reprod BioMed Online. 2016;32(5):503-12.
- Tal R, Seifer CM, Khanimov M, et al. High serum Antimullerian hormone levels are associated with lower live birth rates in women with polycystic ovarian syndrome undergoing assisted reproductive technology. Reprod Biol Endocrinol. 2020;18(1):1-8.
- Zakaria AE, Yosef EH, Deif OM, et al. Serum Anti-Mullerian Hormone, Follicle Stimulating Hormone and Antral Follicle Count Measurement as a Prediction for Pregnancy Rates in IVF/ICSI cycle. Egypt J Hosp Med. 2019;77(1):4692-9.
- Rida M. Role of Anti-Müllerian Hormone in Prediction of ICSI Outcome in Sudanese Women with Polycystic Ovaries Syndrome. EC Gynaecol. 2019;8:978-83.
Author Info
Ahmed Mohamed Abd El-Hay*, Mohammed Salah Elsayed Elsokkary and Bassem Aly IslamCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.