Research - (2024) Volume 19, Issue 4
Can we use Anti Mullerian hormone in follicular fluid as a potential biomarker for oocyte and embryo quality during an In-vitro Fertilisation cycle ?
Rabab M. Sorour1*, Osama Fekry2, Tamer Taha1, Sondos Salem1 and Mohamed A. ElNoury2Received: 04-Nov-2024, Manuscript No. gpmp-25-161649; Editor assigned: 05-Nov-2024, Pre QC No. P-161649; Reviewed: 20-Nov-2024, QC No. Q-161649; Revised: 30-Nov-2024, Manuscript No. R-161649; Published: 30-Dec-2024
Abstract
Background: Anti-Müllarian Hormone (AMH) is a member of the transforming growth factor-β superfamily. It is a yield of granulosa cells lining the FSH dependent growing preantral and small antral follicles. Some authors suggested a relationship between the serum AMH level and oocyte and embryo quality during ovarian stimulation through an IVF cycle.
Material and methods: This prospective observational clinical study was done at Ain Shams University, maternity hospital (IVF Unit), and a private ICSI center from May 2019 to June 2020. Our study used the ALU- QPCR technique and ELISA (Immunotech Beckman Coulter, Marseille, France). To accurately measure the cell-free DNA (cfDNA) level and FF AMH levels respectively, in 35 follicular fluid samples from 15 patients undergoing ICSI.
Results: Our results displayed the range of FFAMH from 1 to 3.8 (ng/ ml). Samples were divided into 3 groups according to the FF AMH levels: (low group, FF AMH<2.1 ng/ mL, patient’s n=5, follicles=10), inter mediate group, FF AMH=2.1-3.6 ng/mL, patient’s n=6, follicles=14), (high group, FF AMH>3.6 ng/mL, patient’s n=4, follicles=11) (Tab. 1). FF AMH levels have no correlation with the embryo quality, cleavage, fragmentation, number of blastomeres on day 3 after fertilization (r=4.348, r=3.263, r=4.348, r=6.269; P=0.114, P=0.196, P=0.114, P=0.180 respectively) (Tab. 2.). Our data showed no significant correlation between individual follicular fluid AMH level, cell free DNA (cfDNA) (r=0.2920, P=0.088) (Tab. 3.) (Fig. 1.). However, Comparing the characteristics and ICSI outcomes among the low, intermediate, and high groups, no significant differences observed except for basal FSH, and numbers of oocytes retrieved. Our study found that the level of cfDNA in follicular fluid is strongly associated with the quality of embryos on Day 3.
Conclusions: Our study results showed no significant correlation between follicular fluid AMH level, oocyte quality or embryo quality. In addition, there is no significant correlation between follicular fluid AMH levels, cell free DNA (cfDNA). Therefore, Individual FF AMH may not be used as a biomarker to judge the oocyte or embryo outcomes during an ICSI cycle.
Keywords
Follicular fluid AMH; Cell-free DNA; Oocytes; Embryo; Blastomeres
Abstract
Background: Anti-Müllarian Hormone (AMH) is a member of the transforming growth factor-β superfamily. It is a yield of granulosa cells lining the FSH dependent growing preantral and small antral follicles. Some authors suggested a relationship between the serum AMH level and oocyte and embryo quality during ovarian stimulation through an IVF cycle.
Material and methods: This prospective observational clinical study was done at Ain Shams University, maternity hospital (IVF Unit), and a private ICSI center from May 2019 to June 2020. Our study used the ALU- QPCR technique and ELISA (Immunotech Beckman Coulter, Marseille, France). To accurately measure the cell-free DNA (cfDNA) level and FF AMH levels respectively, in 35 follicular fluid samples from 15 patients undergoing ICSI.
Results: Our results displayed the range of FFAMH from 1 to 3.8 (ng/ ml). Samples were divided into 3 groups according to the FF AMH levels: (low group, FF AMH<2.1 ng/ mL, patient’s n=5, follicles=10), inter mediate group, FF AMH=2.1-3.6 ng/mL, patient’s n=6, follicles=14), (high group, FF AMH>3.6 ng/mL, patient’s n=4, follicles=11) (Tab. 1). FF AMH levels have no correlation with the embryo quality, cleavage, fragmentation, number of blastomeres on day 3 after fertilization (r=4.348, r=3.263, r=4.348, r=6.269; P=0.114, P=0.196, P=0.114, P=0.180 respectively) (Tab. 2.). Our data showed no significant correlation between individual follicular fluid AMH level, cell free DNA (cfDNA) (r=0.2920, P=0.088) (Tab. 3.) (Fig. 1.). However, Comparing the characteristics and ICSI outcomes among the low, intermediate, and high groups, no significant differences observed except for basal FSH, and numbers of oocytes retrieved. Our study found that the level of cfDNA in follicular fluid is strongly associated with the quality of embryos on Day 3.
Conclusions: Our study results showed no significant correlation between follicular fluid AMH level, oocyte quality or embryo quality. In addition, there is no significant correlation between follicular fluid AMH levels, cell free DNA (cfDNA). Therefore, Individual FF AMH may not be used as a biomarker to judge the oocyte or embryo outcomes during an ICSI cycle.
Abbreviations
AMH: Anti-Müllarian Hormone; cfDNA: Circulating cell-free DNA; COH: Controlled Ovarian Hyperstimulation; ET: Embryo Transfer; FF: Follicular Fluid; ICSI: Intracytoplasmic Sperm Injection; IVF: In vitro fertilization; OCCC: Oocyte Corona cumulus complexes
Introduction
Anti-Müllerian Hormone (AMH) is a member of the transforming growth factor-βsuperfamily [1-4]. It has a pivotal role in embryonic sexual differentiation as it was named referring to inhibition of mullerian duct in male fetuses.
The AMH has a crucial role that regulate follicular developmental competence [5-8]. AMH is a yield of granulosa cells lining the FSH dependent growing preantral and small antral follicles [9-12], It represents an estimation of follicle cohort or the residual primordial follicles [9,13].
Josso and his colleagues cleared that the AMH desensitizes the LH receptors on granulosa cell surface [14].
Thanks to low discrepancy in assays during different times of menstrual cycle that allows us to use it as a reliable clinical use to test the ovarian reserve [15-17].
Furthermore, AMH showed a linear decline with advancement of woman age [16], accurate measurements made clinicians trust to use AMH levels replacing any hormonal parameters, for further diverse clinical implication: to asses fertility in general population, detection of diminished or poor ovarian reserve (DOI), (POI), tailoring the stimulation protocol in Polycystic Ovarian Syndrome (PCOS) patients and fertility preservation strategies [18-21].
Materials and Methods
Participants and study design
This prospective observational clinical study was done at Ain Shams University maternity hospital (IVF Unit) and a private ICSI center from May 2019 to June 2020.
The study included 35 follicles from 15 patients recruited for ICSI. The study gained ethical committee approval from the institution review board.
The present study defined by specific inclusion criteria. Monitoring carried out every other day until day 14, with daily monitoring when deemed necessary. When three or more follicles with diameters greater than 16 mm detected, we give patients Human Chorionic Gonadotropin (HCG 10,000IU, IM) via intramuscular injection as a triggering hormone at 8 pm on the same day. Oocytes retrieved 36 hours later.
Fertilization and culture applied to patient selection
Patients between the ages of 18 and 35, diagnosed with tubal factor of infertility with tubal infertility (without hydrosalpinx), male infertility (excluding azoospermia or low fertility rate), or other factors (including unexplained infertility), normal uterine and cervical morphology, and medical health were included.
The study also employed specific exclusion criteria to ensure the validity and integrity of the data obtained. Patients who were above 35 years of age, women diagnosed with endometriosis, patients with severe male factor (azoospermia requiring testicular biopsy or with a previous low fertilization rate), patients with hydrosalpinx, patients whose Follicle-Stimulating Hormone (FSH) levels were greater than 12 MIU, poor endometrium, and poor ovarian response (POR) were excluded. POR defined according to the Bologna consensus criteria based on two of three factors: maternal age ≥ 40 years, previous POR, or an abnormal ovarian reserve test (i.e., Antral Follicular Count (AFC)<5-7 follicles, or Anti-Müllerian Hormone (AMH)<0.5-1.1 ng/mL).
Patients complicated by Ovarian Hyperstimulation Syndrome (OHSS) and those who refused to consent to the use of their data in the study were also excluded. These rigorous criteria ensured that the study participants represented the intended population and minimized potential sources of confounding or bias. All participants in the study provided with a detailed explanation of the purpose and procedures involved in the research prior to their recruitment. After ensuring that the participants understood the study's objectives, written consent obtained from each of them. This consent obtained in a fully informed manner, with all the relevant details about the study disclosed to the participants. Only after they had provided their written consent did they become part of the study.
All procedures were performed in accordance with the ethical standards from the National Institute of Laser Enhanced Sciences (N.I.L.E.S) - Cairo University with the number EC Ref No: 018- 010.
ICSI Stimulation protocol, oocyte retrieval, fertilization procedures, and embryo culture
Each patient was subjected to treatment via a long agonist stimulation protocol that was initiated on cycle day 21 using triptoreline (Decapeptyl Ferring Pharmaceuticals, Germany) at a dosage of 0.05mg/day S.C. An individualized dose of human menopausal gonadotropin 300-450 IU IM daily (HMG 75 IU, Merional, IBSA) was administered to each patient from days 2 to 3 of the subsequent cycle, following confirmation of down-regulation, which was preceded by an examination of E2 levels. The dose tailored to each patient based on the diameter of the follicles detected during follow-up vaginal ultrasound.
After the oocytes retrieved, the granular cells and corona radiata of the cumulus oophorus removed. The maturity of the oocytes evaluated, and they subjected to ICSI treatment. Following fertilization, the zygote was incubated for 18 hours in an IVF nutrient solution at a temperature of 37 ˚C with a 5% CO2 atmosphere. The fertilization status observed at 24 hours, and the nutrient solution was renewed.
In the process of evaluating Oocyte Corona Cumulus Complexes (OCCC), a stereomicroscope utilized to assess their maturation. Each OCCC assigned a grade from zero to four based on specific criteria.
The criteria used to grade the OCCC were as follows: oocytes with a large nucleus in their cytoplasm and a dense corona cumulus layer deemed immature and assigned a grade of "0". Oocytes lacking a cytoplasmic germinal vesicle and polar body and possessing a corona layer smaller than the total size of five oocytes assigned a grade of "1st degree". Oocytes with radially placed crowded coronal cells, cumulus cells spreading to a relatively wider area than the second group, containing a polar body, and easily removed from the cell group when manipulated, assigned a grade of "2nd degree". Oocytes that lost their tight adherence to the coronal cells and had cumulus cells quite scattered but still cellular assigned a grade of "3rd degree". Oocytes with pale cytoplasm and cumulus cells that lost their cellular image and became gelatinous structures in their vicinity assigned a grade of "4th degree" mature in the classification (M2 and Late M2). It is worth noting that M2 oocytes have clear cytoplasm, normal cell size, normal zona pellucida, and a non-fragmented polar body. Late M2 oocytes are post-mature oocytes that have dark cytoplasm with cytoplasmic granulations, abnormalities in the zona pellucida, and fragmented polar bodies. M2 oocytes considered superior to late M2 oocytes.
The following is a summary of the procedures involved in IVF, specifically semen collection, sperm preparation, and embryo culture. Semen collection and sperm preparation involves the careful extraction of semen and the processing of the sample to yield viable sperm for use in fertilization.
Embryo culture begins with the injection of oocytes and proceeds with a check for fertilization and an embryo morphology assessment. Fertilization ascertained by examining morphological indications, including the appearance of two pronuclei and the extrusion of the second polar body.
The embryo's morphology assessed at or near the transfer point, with early embryos analyzed for the total number of blastomeres, the presence of blastomere regularity, and the extent of fragmentation.
• These procedures are critical in the IVF process and require careful attention to detail by all involved parties to ensure optimal outcomes.
Multinucleation compaction of blastomeres as:
Embryo Grading System The following is a list of ratings and their respective descriptions for embryo grading in the context of assisted reproductive technologies:
Grade 1: Good - Fragmentation of less than 10% - 6-8 blastomeres - Stage-specific cell size - No multinucleation.
Grade 2: Fair - Fragmentation between 10% and 25% - 6-8 blastomeres - Stage-specific cell size for most cells - No evidence of multinucleation.
Grade 3: Poor - Severe fragmentation, more than 25% - Cell size not stage-specific - Presence of multinucleation.
Grade 4: Poor - Severe fragmentation, more than 40% - Cell size not stage-specific - Irregular blastomeres in the context of assisted reproductive technologies, embryo grading is an essential factor in determining the viability of embryos for procedures such as in vitro fertilization. The above-described criteria provide a standardized method for assessing embryos and determining their suitability in these procedures [22,23]. The three most viable embryos transferred to the uterus on the third day, between 66-74 hours after insemination. If possible, embryo transfers were done during the second or third day after oocyte collection, when the embryos were in at least the two- cell stage. It is important to note that we only included patients who underwent fresh embryo transfer.
FF collection
Follicular fluid processing and Cell-free DNA extraction and quantification: During oocyte retrieval Follicular fluids aspirated individually without flushing for each patient. Follicle aspirates that were unclear, such as those contaminated with blood, discarded. After collecting the oocytes, the follicular fluid was centrifuged at 3000 g for 15 minutes, and the supernatant was filtered with 0.22 μm filters to eliminate cell debris, including granulosa cells, erythrocytes, and leukocytes. The samples frozen at -80 °C until FFAMH and cfDNA quantification
Cell-free DNA extraction and quantification
Follicular fluid samples were prepared as described above for cell-free DNA extraction and quantification. Briefly, 20 µl of each follicular fluid sample mixed with 20 µl of a buffer containing 25 ml/l Tween 20, 50 mmol/l Tris, and one mmol/l EDTA. The sample then digested with 16 µg of Proteinase K (PK) (Qiagen) at 50 °C for 20 minutes, followed by heat inactivation and insolubilization at 95 °C for 5 minutes. The samples centrifuged at 10000g for 5 minutes, and supernatants collected and stored at -80 °C until cfDNA quantification. CfDNA was quantified by qPCR for human ALU repeats using two primer sets that generate a 115-bp amplicon (ALU115 primers) and a 247- bp amplicon (ALU247 primers), respectively. For each ALUqPCR, 1 µl of each PK-digested follicular fluid sample was added to a reaction mixture (final volume: 10 µl) containing 0.25 µM of forward and reverse primers (ALU115 or ALU247) and 5 µl of 2X LightCycler®480 SYBR Green I master mix (Roche Applied Science, Germany). Follicular fluid cfDNA concentrations calculated based on a standard curve prepared with successive dilutions of genomic DNA. A negative control (without a template) added to each qPCR plate. All measures performed in quadruplicate [23,24].
The following hormonal assays conducted:
FSH, estradiol, and LH. These were measured using Chemiluminescent Microparticle Immunoassay kits (Architect Abbott Lab, IL, and USA). The concentration of E2 in follicular fluid samples determined by Immunochemiluminescence using Cobas e411 kits from Roche Diagnostics.
Progesterone (Cyclogest 400 mg TDS vaginal sup.) administration used in all cases starting on the day of oocyte retrieval.
FF AMH measurements
FF AMH levels were measured using enzyme-linked immunosorbent assay (Immunotech Beckman Coulter, Marseille, France). The measurement range was 1-3.8 ng/ml.
FF AMH groups
Samples divided into 3 groups according to the FF AMH levels: (low group, FF AMH<2.1 ng/ mL, patients n=5, follicles=10), inter mediate group, FF AMH=2.1-3.6 ng/mL, patients n=6, follicles=14), (high group, FF AMH>3.6 ng/mL, patients n =4, follicles=11).
Statistical analysis
Data analyzed with SPSS software version 28.0 (SPSS, Chicago, IL, USA). Data compared by Kruskal-Wallis test, analysis of variance (ANOVA), or chi-square test, as indicated. Correlation between the FF AMH level and day 3 embryo grade determined by bivariate correlation analysis with Pearson’s correlation coefficients. The result considered as significant when the P value was<0.05.
Some authors suggested a relationship between the serum AMH level and oocyte and embryo quality during ovarian stimulation through an IVF cycle [7,25-29]. Some studies cleared the capability of FF AMH in an individual preovulatory follicle to predict embryo implantation [27].
Takahashi and his colleagues concluded that high levels of AMH in follicles measured related to the fertilized oocytes [30].
A recent study revealed a statistically significant correlation between antral follicle count and AMH in follicular fluid in a cohort of PCO patients [31,32] using minimal ovarian stimulation protocols. We aim to investigate the impact of cry storage duration on clinical and neonatal outcomes, emphasizing the importance of considering this factor in the selection of blastocysts for transfer.
Characteristics of the study patients and long agonist stimulation ICSI outcomes
Comparing the characteristics and ICSI outcomes among the low, intermediate, and high groups, no significant differences observed except for basal FSH, and numbers of oocytes retrieved.
Correlation of the FF AMH level with embryo quality FF AMH levels have no correlation with the embryo quality, cleavage, fragmentation, number of blastomeres on day 3 after fertilization (r=4.348, r=3.263, r=4.348, r=6.269; P=0.114, P=0.196, P=0.114, P=0.180respectively) (Tab. 1. and Tab. 2.). Correlation of the FF AMH level with FF CFDNA Our data showed no significant correlation between individual follicular fluid AMH level, cell free DNA (cfDNA) (r=0.2920, P=0.088) (Tab. 3.) (Fig. 1.).
| Variables | Low group (<2.1 ng/mL) (Patients n=5) (Follicles n=10) |
Intermediate group (2.1-3.6 ng/mL) (Patients n=6) (Follicles n=14) |
High group (>3.6 ng/mL) (Patients n=4) (Follicles n=11) |
Kruska l- Wallis test value | p- value (Krusk al- Wallis) |
|---|---|---|---|---|---|
| Age (years) | 29.40 ± 4.56 | 28.00 ± 5.73 | 27.50 ± 2.08 | 0.573 | 0.751 |
| 31 (23-35) | 29 (20-35) | 27.5 (25-30) | |||
| BMI (kg/m2) | 28.80 ± 2.59 | 26.67 ± 5.50 | 26.25 ± 2.99 | 0.969 | 0.616 |
| 29 (25-32) | 27.5 (19-32) | 26 (23-30) | |||
| Infertility | 7.60 ± 2.19 | 6.33 ± 3.08 | 7.25 ± 0.96 | 1.025 | 0.599 |
| duration (years) | 9 (4-9) | 7.5 (2-9) | 7.5 (6-8) | ||
| Serum FSH | 5.42 ± 0.55 | 7.48 ± 1.14 | 8.85 ± 0.94 | 10.727 | 0.005 |
| (mIU/l) | 5.5 (4.8-6) | 7.2 (6.1-9.4) | 8.75 (7.9-10) | ||
| Number of | 6.60 ± 0.55 | 7.67 ± 0.82 | 11.25 ± 3.30 | 10.485 | 0.005 |
| oocytes | 7 (6-7) | 7.5 (7-9) | 10 (9-16) | ||
| Number of days | 14.60 ± 1.14 | 16.00 ± 0.63 | 16.25 ± 1.50 | 5.256 | 0.072 |
| stimulation | 15 (13-16) | 16 (15-17) | 17 (14-17) | ||
| Folliclesize | 21.10 ± 3.21 | 20.71 ± 2.20 | 21.73 ± 1.79 | 1.228 | 0.541 |
| (mm) | 19.5 (18-25) | 20 (18-25) | 22 (19-24) | ||
| Number of | 2.00 ± 0.00 | 2.33 ± 0.52 | 2.75 ± 0.50 | 5.250 | 0.072 |
| embryos | 2 (2-2) | 2 (2-3) | 3 (2-3) | ||
| Immature | 1.50 ± 0.53 | 1.71 ± 0.47 | 1.82 ± 0.40 | 2.476 | 0.290 |
| oocytes | 1.5 (1-2) | 2 (1-2) | 2 (1-2) | ||
| Embryo grade | 1.60 ± 1.34 | 3.00 ± 1.11 | 2.64 ± 1.03 | 4.878 | 0.087 |
| 1 (1-4) | 3 (1-4) | 2 (1-4) | |||
| cfDNA (ng/ml) | 2.47 ± 1.14 | 2.01 ± 1.08 | 1.75 ± 0.96 | 2.339 | 0.311 |
| 2.67 (0.77-3.89) | 1.83 (0.24-3.86) | 1.33 (0.41-3.43) | |||
| Data shown as the mean ± SD and proportion (%) P<0.05 statistically significant BMI: Body Mass Index, cfDNA: cell free DNA |
|||||
Tab. 1. Characteristics of the study patients and long agonist stimulation ICSI outcomes.
| Variables | Low group (<2.1 ng/mL) n (%) | Intermediate group (2.1-3.6 ng/mL) n (%) |
High group (>3.6 ng/mL) n (%) | Pearson Chi- Square | p-value |
|---|---|---|---|---|---|
| Infertility Etiology | |||||
| - Female factor - Male factor - Mixed infertility - Unexplained |
3 (60.0%) | 3 (50.0%) | 2 (50.0%) | ||
| 0 (0.0%) | 1 (16.7%) | 2 (50.0%) | |||
| 1 (20.0%) 1 (20.0%) |
1 (16.7%) 1 (16.7%) |
0 (0.0%) 0 (0.0%) |
4.396 | 0.623 | |
| Embryo quality - Top quality embryos (Grades 1 and 2) - Low quality embryos (Grades 3 and 4) |
4 (80.0%) 1 (20.0%) |
4 (28.6%) 10 (71.4%) |
6 (54.5%) 5 (45.5%) |
4.348 | 0.114 |
| Cleavage - Early cleavage - No early cleavage |
0 (0.0%) 5 (100.0%) |
5 (35.7%) 9 (64.3%) |
5 (45.5%) 6 (54.5%) |
3.263 | 0.196 |
| Fragmentation rate - ≤ 25% - >25% |
4 (80.0%) 1 (20.0%) |
4 (28.6%) 10 (71.4%) |
6 (54.5%) 5 (45.5%) |
4.348 | 0.114 |
| Number of Blastomeres - <6 cells - 6-8 cells - >8 cells |
1 (20.0%) 0 (0.0%) 4 (80.0%) |
6 (42.9%) 5 (35.7%) 3 (21.4%) |
5 (45.5%) 2 (18.2%) 4 (36.4%) |
6.269 | 0.180 |
Tab. 2. Correlation of the FF AMH level in groups with embryo quality.
| Follicular Fluid AMH (ng/ml) | Variables | r | P-value |
| Age (years) | -0.326 | 0.235 | |
| BMI (kg/m2) | -0.201 | 0.473 | |
| Infertility duration (years) | -0.249 | 0.371 | |
| Serum FSH (IU/l) | 0.942 | <0.001 | |
| Total dose of gonadotropins | -0.758 | 0.001 | |
| Number of days stimulation | 0.687 | 0.005 | |
| Follicle size (mm) | 0.076 | 0.666 | |
| Immature oocytes | 0.269 | 0.118 | |
| Embryo grade | 0.267 | 0.153 | |
| cfDNA (ng/ml) | 0.292 | 0.088 |
Tab. 3. Correlations of the FF AMH level with characteristics and outcomes of patients.
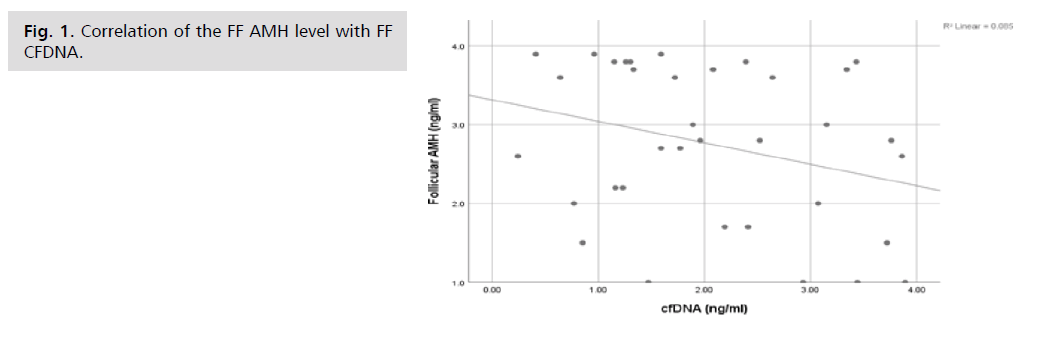
Fig. 1. Correlation of the FF AMH level with FF CFDNA.
Discussion
Our study results showed no significant correlation between follicular fluid AMH level, oocyte quality or embryo quality.
We have measured FF AMH level in leading follicles after ovulation triggering as a part of an ICSI –after using long agonist protocol.
Some authors have found a correlation between FF AMH at ovum pick up and oocyte quality [30,33].
One retrospective study concluded the ability to use FFAMH as a predictive index for successful oocyte fertilization [30,33].
Other authors compared serum and pooled FF AMH and they suggested a potential use of follicular AMH to predict embryo implantation, but they failed to show a difference between low FFAMH values and oocyte or embryo quality [28,34].
Upon reviewing the literature, we have found authors with an opposite view; they showed an inverse correlation between high levels of pooled FF AMH and the oocyte developmental competence, embryo quality and clinical pregnancy rate [35-38].
The individual follicular fluid represents an intimate developmental milieu for an oocyte competence, fertilization potential and subsequently, corresponding embryo rather than pooled follicular fluid which is nonspecific to a certain oocyte. It was known to us that there is a negative correlation between the granulosa cells apoptotic rates and the development of top-quality embryos [39,40].
In addition, there is a stage specific oocytes regulation of AMH mRNA expression that coordinates follicular competence and there is higher AMH mRNA expression in cultured cumulus cell of antral follicles more than mural granulosa cells in mice model [41,42].
Conclusion
Our study results showed no significant correlation between follicular fluid AMH level, oocyte quality or embryo quality.
In addition, there is no significant correlation between follicular fluid AMH levels, cell free DNA (cfDNA).
Therefore, Individual FF AMH may not be used as a biomarker to judge the oocyte or embryo outcomes during an ICSI cycle.
Acknowledgements
We would like to especially thank the staff of Ain Shams University hospital - IVF unit.
Funding
Not applicable.
Availability of Data and Materials
All data generated or analyzed during this study are available from correspondence on request.
Ethics Approval
All procedures were performed in accordance with the ethical standards from the National Institute of Laser Enhanced Sciences (N.I.L.E.S) - Cairo University with the number EC Ref No: 018- 010.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
References
- Josso N, di Clemente N. TGF-β family members and gonadal development. Trends Endocrinol Metab. 1999;10(6):216-222.
- Chattha AJ, Salama M, Jayasinghe Y. Fertility preservation in the pediatric population. Front Endocrinol. 2023;14:1149532.
- Picard JY, Benarous R, Guerrier D, et al. Cloning and expression of cDNA for anti-müllerian hormone. Proc Natl Acad Sci USA. 1986;83(15):5464-5468.
- Yildiz S, Moolhuijsen LM, Visser JA. The Role of Anti-Müllerian Hormone in Ovarian Function. Semin Reprod Med. 2024. Thieme Medical Publishers, Inc..
- Knight PG, Glister C. Local roles of TGF-β superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78(3-4):165-183.
- Lim C. Anti-Müllerian Hormone (AMH) Signalling in the Ovary (Doctoral dissertation, University of Otago).
- Seifer DB, MacLaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539-546.
- Xu F, Bagnjuk K, Marti-Gutierrez N, et al. Reduced anti-Müllerian hormone action in cumulus-oocyte complexes is beneficial for oocyte maturation without affecting oocyte competence. Front Endocrinol. 2024;15:1365260.
- Hassan J. Delineating cell types and toxicity in human ovaries from birth to sexual maturity. Karolinska Institutet (Sweden); 2024.
- Hudson PL, Dougas I, Donahoe PK, et al. An immunoassay to detect human mullerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab. 1990;70(1):16-22.
- Iwase A, Hasegawa Y, Tsukui Y, et al. Anti-Müllerian hormone beyond an ovarian reserve marker: the relationship with the physiology and pathology in the life-long follicle development. Front Endocrinol. 2023;14:1273966.
- Van Rooij IA, Broekmans FJ, Te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065-3071.
- Monniaux D, Clément F, Dalbiès-Tran R, et al. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link?. Biol Reprod. 2014;90(4):85-81.
- Josso N, Racine C, di Clemente N, et al. The role of anti-Müllerian hormone in gonadal development. Mol Cell Endocrinol. 1998;145(1-2):3-7.
- Iwase A, Nakamura T, Osuka S, et al. Anti-Müllerian hormone as a marker of ovarian reserve: What have we learned, and what should we know?. Reprod Med Biol. 2016;15:127-136.
- Moolhuijsen LM, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361-3373.
- Silva MS, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic–pituitary–gonadal axis and neuroendocrine development. Cell Mol Life Sci. 2021;78(1):1-6.
- Iwase A, Osuka S, Goto M, et al. Clinical application of serum anti‐Müllerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res. 2018;44(6):998-1006.
- Jaffar M, Ahmad SN, Ashraf M, et al. Geographical Diversity in the Age Specific Anti Müllerian Hormone Levels in Infertile Women: A Hospital based Cohort Study. J Hum Reprod Sci. 2023;16(1):29-35.
- Nguyen DK. The Roles of Anti-Müllerian Hormone in Individualised Fertility Treatment. PhD Thesis. 2023.
- De Vet A, Laven JS, de Jong FH, et al. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357-362.
- Malhotra K, Malhotra J, Malhotra N, et al. Embryo Morphology and Embryoscopy. InAtlas of Assisted Reproductive Technologies,Singapore: Springer Nature Singapore. 2023: 205-212.
- Scalici E, Traver S, Molinari N, et al. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014;29(12):2661-2669.
- Terp SK, Pedersen IS, Stoico MP. Extraction of cell-free DNA: evaluation of efficiency, quantity, and quality. J Mol Diagn. 2024;26(4):310-319.
- Ebner T, Sommergruber M, Moser M, et al. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022-2026.
- Heidary Z, Masoumi M, Dashtkoohi M, et al. The association of AMH level with the number and quality of oocytes in women undergoing IVF/ICSI: a single-center study. J Reprod Infertil. 2024;25(1):38.
- Irez T, Ocal P, Guralp O, et al. Different serum anti-Müllerian hormone concentrations are associated with oocyte quality, embryo development parameters and IVF-ICSI outcomes. Arch Gynecol Obstet. 2011;284:1295-1301.
- Sacha CR, Chavarro JE, Williams PL, et al. Follicular fluid Anti-Müllerian hormone (AMH) concentrations and outcomes of in vitro fertilization cycles with fresh embryo transfer among women at a fertility center. J Assist Reprod Genet. 2020;37:2757-2766.
- Tramišak Milaković T, Panić Horvat L, Čavlović K, et al. Follicular fluid anti-Müllerian hormone: a predictive marker of fertilization capacity of MII oocytes. Arch Gynecol Obstet. 2015;291:681-687.
- Takahashi C, Fujito A, Kazuka M, et al. Anti-Müllerian hormone substance from follicular fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil Steril. 2008;89(3):586-591.
- Chen Y, Ye B, Yang X, et al. Predicting the outcome of different protocols of in vitro fertilization with anti-Muüllerian hormone levels in patients with polycystic ovary syndrome. J Int Med Res. 2017;45(3):1138-1147.
- Delamuta LC, Fassolas G, Dias JA, et al. Antimüllerian hormone levels and IVF outcomes in polycystic ovary syndrome women: a scoping review. JBRA Assist Reprod. 2024;28(2):299.
- Moreira MV, Vale-Fernandes E, Albergaria IC, et al. Follicular fluid composition and reproductive outcomes of women with polycystic ovary syndrome undergoing in vitro fertilization: a systematic review. Rev Endocr Metab Disord. 2023;24(6):1045-1073.
- Fanchin R, Mendez Lozano DH, Frydman N, et al. Anti-Mullerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92(5):1796-1802.
- Cupisti S, Dittrich R, Mueller A, et al. Correlations between anti-müllerian hormone, inhibin b, and activin ain follicular fluid in ivf/icsi patients for assessing the maturation and developmental potential of oocytes. Eur J Med Res. 2007;12:604-608.
- Dai M, Hong L, Yin T, et al. Disturbed follicular microenvironment in polycystic ovary syndrome: relationship to oocyte quality and infertility. Endocrinol. 2024;165(4):bqae023.
- Mehta BN, Chimote MN, Chimote NN, et al. Follicular-Fluid Anti-Mullerian hormone (FF AMH) is a plausible biochemical indicator of functional viability of oocyte in conventional in vitro fertilization (IVF) cycles. J Hum Reprod Sci. 2013;6(2):99-105.
- Nakahara K, Saito H, Saito T, et al. Incidence of apoptotic bodies in membrana granulosa of the patients participating in an in vitro fertilization program. Fertil Steril. 1997;67(2):302-308.
- Zeuner A, Müller K, Reguszynski K, et al. Apoptosis within bovine follicular cells and its effect on oocyte development during in vitro maturation. Theriogenology. 2003;59(5-6):1421-1433.
- Nakahara K, Saito H, Saito T, et al. The incidence of apoptotic bodies in membrana granulosa can predict prognosis of ova from patients participating in in vitro fertilization programs. Fertil Steril. 1997;68(2):312-317.
- Münsterberg A, Lovell-Badge R. Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113(2):613-624.
- Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201-208.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Rabab M. Sorour1*, Osama Fekry2, Tamer Taha1, Sondos Salem1 and Mohamed A. ElNoury22Department of Medical Application of Laser, National Institute of Laser Enhanced Sciences, Cairo University, and Giza, Egypt
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.