Research Article - (2022) Volume 17, Issue 1
Two Different Doses of Self-administered Vaginal Misoprostol for Successful Copper Intrauterine Device Insertion in Parous Women Previously Delivered by Cesarean Section- A Double Blinded Randomized Clinical Trial
Ahmed Alanwar*, Sherif Naguip, Alaa El-Ghanam and Haitham El-SabaaReceived: 04-Feb-2022, Manuscript No. gpmp-22-53526; Editor assigned: 05-Feb-2022, Pre QC No. P-53526; Reviewed: 10-Feb-2022, QC No. Q-53526; Revised: 21-Feb-2022, Manuscript No. R-53526; Published: 29-Mar-2022
Abstract
Objective: The aim of the study was to compare the efficacy and safety of misoprostol 200 mcg plus placebo vs. 400 mcg administered vaginally prior to IUCD insertion regarding the success and ease of insertion among parous women previously delivered by cesarean section beside the rate of occurrence of adverse effects.
Methods: This double blind randomized controlled clinical trial was conducted at Ain Shams University Maternity Hospital during the period from January 2020 till July 2020. One hundred parous women previously delivered by caesarean section were randomized into 2 groups; group (1): 50 women received misoprostol 400 mcg vaginally 3 hours prior to IUCD insertion and group (2): 50 women received misoprostol 200 mcg plus placebo vaginally 3 hours prior to IUCD insertion.
Results: There was insignificant difference between both groups; VAS ranged between 1 and 5 with a mean value of 2.16 ± 0.93 in group 1 and between 1 and 5 with a mean value of 2.55 ± 1.21 in group 2. There was insignificantly different between both groups. However, group 1 showed significantly lower need of analgesia than group 2 (P = 0.004). Successful IUD insertion was insignificantly different between both groups. Woman's level of satisfaction was insignificantly different between both groups. All side effects were insignificantly different between both groups except abdominal cramping and shivering were significantly lower in group 2 than in group 1.
Conclusion: We recommend using the lowest dose of misoprostol (200 mcg) prior to IUCD insertion.
Keywords
Intrauterine contraceptive device; Cesarean section; Misoprostol
Introduction
The intrauterine contraceptive device (IUCD) is highly regarded as both highly efficient and exceptionally safe as a long-acting reversible contraception method. In terms of long-acting reversible contraception, intrauterine contraceptive devices (IUCDs) are considered secure, dependable, and trustworthy options [1].
The widespread utilization of IUCD can be attributed to their numerous benefits such as reversible fertility immediately after removal, freedom from daily reminders, absence of hormonal impacts, compatibility with breastfeeding, no disruption to sexual activities and medicines. However, despite these advantages, IUCDs may not always yield successful outcomes [2].
Women's reluctance to use IUCDs primarily stems from their fear of experiencing pain during insertion. For healthcare professionals the obstacles to use include fear of causing pain with the procedure and difficulties during the procedure that could end in failure of insertion. Insertion-associated pain is related to speculum insertion, tenaculum traction on the cervix, cervical immaturity, sounding of the uterus, passing of the insertion tube throughout the cervix and the subsequent device positioning inside the uterine cavity [3].
Misoprostol is a cost-effective synthetic prostaglandin estrone analog. It can be taken vaginally or orally the night before and, and if deemed necessary, repeated in the morning prior to minimally invasive gynecological operations like hysteroscopy, facilitating cervical softening. Nevertheless, the administration of misoprostol is linked to possible side effects such as abdominal cramps, nausea, shivering, uterine bleeding, vomiting, and diarrhea [4].
This study aims to evaluate and compare the effectiveness and safety of misoprostol 200 mcg plus placebo vs. 400 mcg administered vaginally prior to IUCD insertion regarding the success and ease of inserting process among parous women previously delivered by cesarean section beside the rate of occurrence of adverse effects.
Materials and Methods
Design and setting
A double blind randomized controlled clinical trial was accomplished on women seeking intra uterine device insertion at Ain Shams University Maternity Hospital (Family planning clinic) from January 2020 till July 2020.
Participants
During the study period, all women visiting the family planning clinic and meeting the following criteria are eligible for IUCD insertion: women at reproductive age group between 18 - 45 years old, parous women previously delivered by cesarean section. Timing of insertion at the last day of menstruation, during puerperium or 2 weeks after abortion. Furthermore, it is required that women have not taken any analgesics within the 24 hours preceding the IUCD insertion, and they should meet the eligibility criteria for IUCD insertion outlined by the World Health Organization (WHO), with no contraindications present.
Exclusion criteria included nulligravidae, previous vaginal delivery, women with contraindications for misoprostol use (pregnancy, prostaglandin allergy), women with a contraindication for IUCD insertion (e.g., less than sex weeks post-partum, gynecologic malignancy, unexplained uterine bleeding, active cervicitis or vaginitis, uterine fibroids or other irregularities, PID or puerperal sepsis history). Women on anticoagulant therapy or having any coagulopathy, uterine fibroid causing cavity distortion, anatomical anomaly leading to cavity distortion, current purulent cervicitis (gonorrhea or chlamydia), current pelvic inflammatory disease, pelvic tuberculosis, puerperal sepsis, immediately after septic abortion, cancer cervix and cancer endometrium.
Sample Size
The study was conducted on (100) women. They were subdivided into 2 groups: Group 1 (control): 50 women received misoprostol 400 mcg (Misotac ®, Sigma, SAE, Egypt) (2 tablets) vaginally 3 hours prior to IUCD insertion. Group 2 (experimental): 50 women received misoprostol 200 mcg plus placebo vaginally 3hours prior to IUCD insertion (the placebo tablet has the same color, size and shape of tablet of misoprostol). The necessary sample size was determined utilizing the IBM© Sample Power© Software (IBM© Corp., Armonk, NY, USA). The principal measure of interest is the success rate for IUCD insertion. In a previous study (5), it was documented that the success rate following previous IUCD insertion failure was 61.9% when adopting placebo or 87.5% pre-emptive vaginal misoprostol. To recognize a significant difference among both groups regarding IUCD insertion success rate, 100 patients sample size is estimated. This would involve equally randomizing 50 patients into each study group, providing a statistical power of 80% (type II error of 0.2) with a two-sided chi-squared test at a 95% confidence level (type I error of 0.05). Under the null hypothesis, it is supposed that the success rate in both groups is equal and set at 61.9%. Conversely, under the alternative hypothesis, the success rate is hypothesized to be either 61.9% with a placebo or 87.5% with vaginal misoprostol.
Randomization
Randomization was accomplished utilizing a computer-generated randomization sheet prepared with MedCalc© version 13. To ensure allocation concealment, sealed opaque envelopes were utilized. These envelopes were handed over to a third party (a nurse) responsible for assigning women to their respective study arms. Each woman was invited to pull out an envelope. According to the number inside her envelope, women was allocated to either group 1 or group 2 according to a computer-generated random list.
Ethical consideration
The study was started after the approval of Research Ethical Committee, Faculty of Medicine, Ain Shams University. Prior to enrollment in the study, all participants provided informed consent. The purpose and procedures of the study were thoroughly explained before seeking the consents. The investigator ensured that the written and signed informed consent was attained from all subjects commencing any study-specific procedures.
Interventions
Participants was distributed equally and randomly into two groups: Group 1 (control): 50 women to who received two misoprostol (Misotac®, Sigma, SAE, Egypt) tablets (400 mg) vaginally 3 h prior to IUCD inserting. The tablets were inserted as deeply as possible, and the participants were instructed to maintain supine situation for thirty minutes. Group 2 (experimental): 50 women to whom one tablet (200 mg) of misoprostol (Misotac®, Sigma, SAE, Egypt) plus placebo was administered vaginally 3 h before IUCD insertion.
All women had their copper IUCD (a T380A [Copper T 380A, ®, Egypt]). IUCD insertion was considered failed if we are unable to go through the internal cervical os using the uterine sound, metallic dilator number 3 and an os Finder, which has a gradually widening plastic tip measuring 1.75 mm to 3.8 mm in diameter.
The involved women were asked to sign an informed consent and was received a sealed opaque envelope with the medication or placebo. The women were provided with instructions to insert vaginally two tablets of misoprostol 200 mg or 200mcg plus placebo hat had been soaked in 5 ml saline 3hr prior to returning to the clinic.
Outcomes
The primary outcome was the ease of IUCD insertions. Difficulty of IUCD insertion was measured by determining if Hegar dilators of 4 mm diameter or smaller could pass through the internal cervical os without resistance. The amount of resistance met when using dilators, and whether any dilation of the cervix was required, was both documented. The investigator also judged and classified the level of difficulty of the IUCD insertion as ‘easy’, ‘moderate’ or ‘difficult’ according to the resistance felt at the internal cervical os during the procedure. This subjective assessment of insertion difficulty was based on the resistance experienced by the inserting clinician when passing through the internal cervical os.
Secondary outcome included uterine or cervical perforation, vasovagal responses (nausea, vomiting, and dizziness), heavy bleeding, syncope, partial- or complete expulsion, pain degree experienced while insertion, and the degree of challenge encountered when placing IUCD.
Pain was assessed with a visual analog scale (VAS) pain score reported by participants during IUCD insertion. Pain score was measured with a VAS with a 10 cm horizontal straight line with 0 cm corresponds to no pain and 10 cm to the worst pain. VAS ratings were categorized as 0 no pain, 1-3 (mild pain), 4-6 (average pain) and 7-9 (severe pain), and 10 (very severe pain) an individual can experience.
The treatment of side effects was in the form of: Uterine and cervical perforation: hospital admission, conservative treatment for 24 hours with antibiotics coverage. Heavy Bleeding: local examination and ultrasound then treatment of the cause e.g., Tranexamic acid. Vasovagal like reactions: analgesics, intravenous fluids, and positive inotropes, with observation of vital data. Syncope: Airway, Breathing, Circulatory assessment and management of the cause. Partial or Total expulsion: Remove IUD. Pain and struggle of IUCD insertion: Counseling, proper timing of IUD insertion (during menstruation), NSAIDs 1hr before insertion and examination for perforation.
The participants also scored side effects of misoprostol or placebo. In this assessment, a checkbox was marked for each side effect, indicating whether it was mild, moderate, or severe. The side effects that were evaluated include abdominal cramping, nausea/vomiting, headache, fever (temperature ≥ 38.08Ë?C), shivering, and diarrhea. The participant completed this side-effect form prior to the insertion of IUCD to certify that side effects from medication/placebo would not be confused with insertion-related side-effects.
Every patient underwent a routine check-up six weeks following IUCD insertion. At this visit, vaginal ultrasound and/or examination were accomplished. IUCD infections and expulsions were documented.
Elimination of bias
All IUD insertions and observation of study outcome was done by the same doctor. All procedures were done by supervisors and experts.
Statistical analysis
Data was collected and organized before being analyzed with IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY). Numerical data that followed a normal distribution was presented as mean and standard deviation (SD), while skewed data was presented as median and interquartile range. Qualitative data was expressed as numbers and percentages. To compare normally distributed numerical data, the unpaired Student t-test was employed, while the Mann-Whitney U test was utilized for skewed data comparisons. When dealing with categorical data, the chi-square test or Fisher's exact test, if suitable, were employed. A two-sided p-value <0.05 was regarded significant in statistical perspective.
Results
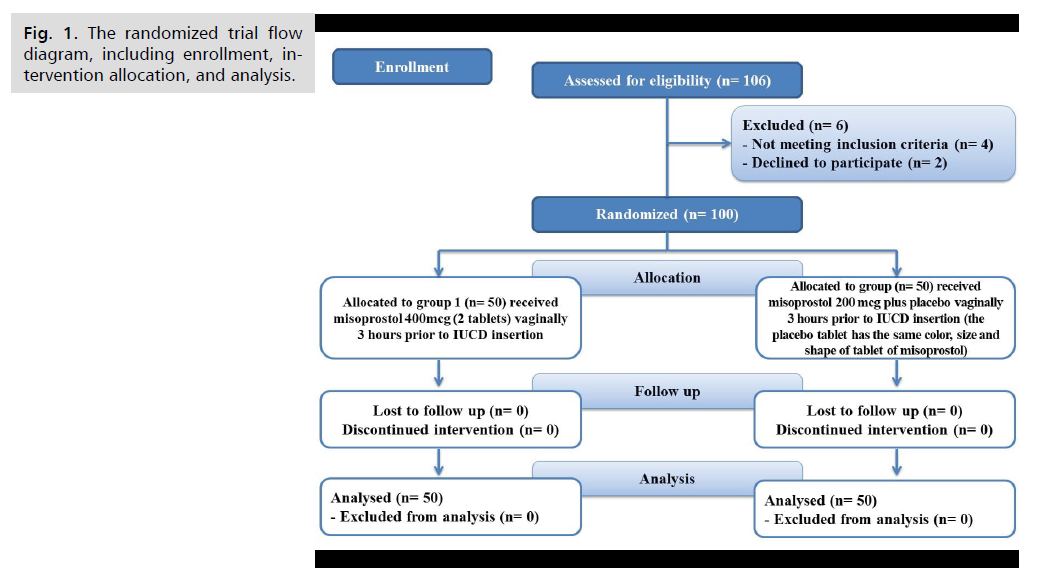
In this study, 106 patients were assessed for eligibility, 4 patients did not meet the inclusion measures, and two cases chose not to participate. The remaining 100 patients were randomly allocated into two groups (50 patients in each one). All of them were followed up and statistically analyzed (Fig. 1.).
Fig 1. The randomized trial flow diagram, including enrollment, intervention allocation, and analysis.
Tab. 1. showed patients’ characteristics in both groups.
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
value | |
|---|---|---|---|---|
| Age (years) | Mean ± SD | 30.8 ± 4.64 | 29.98 ± 4.58 | 0.376 |
| Range | 24 - 38 | 23 - 43 | ||
| BMI (kg/m2) | Mean ± SD | 26.64 ± 3.99 | 27.85 ± 4.61 | 0.164 |
| Range | 20.2 - 34.4 | 20.1 - 33.9 | ||
| Parity | Mean ± SD | 2.08 ± 0.94 | 1.92 ± 0.88 | 0.382 |
| Range | 1 - 4 | 1 - 4 | ||
| Previous miscarriages | 10 (20.0%) | 6 (12.0%) | 0.275 | |
| Previous CS | 50 (100.0%) | 50 (100.0%) | --- | |
| Previous use of contraceptives | 22 (44.0%) | 19 (38.0%) | 0.542 | |
| Previous insertion of IUD | 15 (30.0%) | 18 (36.0%) | 0.523 | |
Tab.1. Patients’ characteristics in both groups.
Tab. 2. showed that visual analog scale (VAS) was insignificantly different between both groups.
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
P value | |
|---|---|---|---|---|
| VAS | Mean ± SD | 2.16 ± 0.93 | 2.55 ± 1.21 | 0.074 |
| Range | 1 - 5 | 1 - 5 | ||
Tab. 2. Visual Analog Scale (VAS) in both groups.
Regarding Tab. 3. group 1 showed significantly lower ease score (ES) in group 1 compared to group 2 (P <0.05).
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
P value | |
|---|---|---|---|---|
| ES | Mean ± SD | 1.6 ± 0.67 | 4 ± 0.99 | <0.001* |
| Range | 1 - 4 | 2 - 6 | ||
Tab. 3. Ease Score (ES) in both groups.
Tab. 4. showed that successful IUD insertion was insignificantly different between both groups.
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
P value | |
|---|---|---|---|---|
| Successful IUD insertion | Succeeded | 48 (96.0%) | 47 (94.0%) | 0.477 |
| Failed | 2 (4.0%) | 3 (6.0%) | ||
Tab. 4. Successful IUD insertion in both groups.
Tab. 5. showed that woman's level of satisfaction was insignificantly different between both groups.
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
P value | |
|---|---|---|---|---|
| Woman's level of satisfaction | Mean ± SD | 8.33 ± 0.80 | 8.04 ± 0.82 | 0.083 |
| Range | 7 - 9 | 6 - 9 | ||
Tab. 5. Woman’s level of satisfaction in both groups.
Regarding Tab. 6. group 1 showed significantly lower need of analgesia than group 2 (P = 0.004).
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
P value | |
|---|---|---|---|---|
| Needing for analgesia | Analgesia needed | 5 (8.3%) | 7 (11.7%) | 0.004* |
| Analgesia not needed |
45 (75.0%) | 43 (71.7%) | ||
Tab. 6. Needing for analgesia in both groups.
Tab. 7. showed that all side effects were insignificantly different between both groups except abdominal and shivering were significantly lower in group 2 than group 1. (P <0.05).
| Variables | Group 1 (n = 50) |
Group 2 (n = 50) |
P value | |
|---|---|---|---|---|
| Side effects | Uterine perforation | 0 (0.0%) | 0 (0.0%) | -- |
| Abdominal cramping | 16 (26.7%) | 5 (8.3%) | 0.004* | |
| Shivering | 8 (16.0%) | 1 (2.0%) | 0.031* | |
| Nausea and vomiting | 3 (6.0%) | 1 (2.0%) | 0.617 | |
| Headache | 1 (2.0%) | 0 (0.0%) | 1 | |
Tab. 7. Side effects in both groups.
Discussion
Fear of discomfort during insertion is among the primary causes for women's limited use of intrauterine contraceptive devices (IUCDs) [6].
The insertion of the speculum, tenaculum uterus sounding, traction on the cervix, insertion tube passage throughout the cervix, and instrument insertion into the uterine cavity are all linked with discomfort [7].
Misoprostol, a synthetic prostaglandin estrone analogue, has been used to aid cervical softening before minimally invasive gynecological procedures like hysteroscopy [8].
A clinical trial was conducted at Ain Shams University Maternity Hospital, employing a double-blind randomized controlled design to appraise and compare the effectiveness and safety of the vaginal administration of misoprostol 200 mcg plus placebo vs. 400 mcg before IUCD inserting regarding the successful and effortlessness inserting procedure among parous women besides the incidence of adverse effects. One hundred parous women previously delivered by cesarean section were randomized into 2 equal groups; group (1): 50 women received misoprostol 400 mcg (2 tablets) vaginally 3 hours prior to IUCD insertion and group (2): 50 women received misoprostol 200 mcg plus placebo vaginally 3 hours prior to IUCD insertion (the placebo tablet has the same color, size and shape of tab of misoprostol).
Regarding baseline patient’s characteristics (Age, BMI, parity, previous miscarriages, previous CS, previous use of contraceptives and previous insertion of IUD):
Edelman et al. [9] agreed with the present data and stated that there were no statistically significant differences in baseline characteristics as age, BMI, history of pregnancies and abortions between the two study groups. The study investigated the impact of administering prophylactic misoprostol before IUD placing in women who have not given birth. Forty nulliparous women of reproductive age and wished to use an IUD for contraception were included in this study. They were randomly assigned to receive either 400 mcg of buccal misoprostol or a placebo 90 minutes before the insertion of the IUD.
Regarding visual analog scale (VAS): Espey et al. [10] disagreed with the present data and stated that no significant differences were reported by providers regarding IUD inserting ease between groups (P =.54). Both providers in the misoprostol and placebo groups found it relatively easy to place the IUD in nulliparous women, with a mean ease of insertion score of 2.2 ± 2.2 and 2.5 ± 2.2 (P = .54), respectively, based on the VAS, this disagreement was due to lack of placebo group in current study.
Mansy [11] agreed with the present data, emphasizing the uterine sounding ease in the misoprostol group. Among the cases in the misoprostol group, 43 (86%) cases had successful insertion, 27(62.8%) reported easy sounding, 16 (37.2%) encountered difficulty and 7 (14%) experienced failed sound insertion. there were 11 (22%) cases of failed insertion, 14 (35.9%) cases of difficult insertion, and 25 (64.1%) cases of easy uterine sound insertion. The calculated p-value of 0.577 indicated no statistically statistical differences among both groups. He assessed sublingual misoprostol effect in reduction of pain and facilitation of IUD insertion in women who had not previously undergone vaginal delivery. This study was designed as a double blinded randomized controlled trial included 400 cases, comparing sublingual 200 mg misoprostol with placebo to facilitate IUD insertion. Also, no statistically significant differences regarding pain decrease when utilizing misoprostol before insertion of the IUD. .
Regarding successful IUD insertion: Dijkhuizen et al. [12] Dijkhuizen et al. [12] agreed with the present data and stated that three insertions failed, two in the misoprostol group and one in the placebo group P= 0.59. Most IUDs were placed during the first attempt: 88 (88%) in the misoprostol group (data for 100 patients) vs. 89 (94.7%) in the placebo group (data for 94 patients; P= 0.13).
Scavuzzi et al. [13] disagreed with the present data and stated that significant differences were found between the groups for all the immediate end points evaluated, with less difficulty in inserting the IUD and less risk of cervical dilatation ≤4 mm when misoprostol was used prior to insertion that was due to current study didn’t include placebo group.
Regarding woman's level of satisfaction: Ibrahim et al. [14] agreed with the present data and stated that patients in both groups were satisfied about their experience, with no significant difference between the groups.
El-Gawad et al. [15] disagreed with the present data and stated that satisfaction was significantly more frequent among Misoprostol group while insertion complications were non-significantly less frequent among Misoprostol group. that was due to current study didn’t include placebo group.
Regarding side effects: Dijkhuizen et al. [12] ddisagreed with the present data and stated that they were significantly more frequent in the misoprostol group: 56 participants (56.6%) who received misoprostol experienced any kind of side effect compared with 39 (42.4%) in the placebo group (P= 0.05). The most common side-effect was cramping in the abdomen (38.2%). Fever (temperature ≥ 38.08Ë?C) did not occur in the misoprostol group, whereas 3.3% of patients in the placebo group experienced fever. Other side- effects included itching, exanthema, sweating and dysuria, did not differ between groups (P = 0.48). In general, all of the side-effects were mild.
Scavuzzi et al. [13] agreed with the present data and stated that there were no significant differences between the groups in relation to complications during IUD insertion. The frequency of bleeding, vasovagal reaction, cramps, nausea, vomiting and insertion failures was similar in both groups. No cases of uterine perforation occurred in either group. There were no significant differences in the frequency of the majority of the immediate side effects such as nausea, vomiting, hyperthermia and diarrhea, evaluated prior to IUD insertion. Nevertheless, there was a significant increase in cramps with the prior use of misoprostol compared with placebo. In relation to the side effects evaluated 24 h after IUD insertion, no significant differences were found between the misoprostol and placebo groups.
Ibrahim et al. [14] agreed with the present data and stated that there were no significant insertion-related complications in either group (infection, perforation or excessive vaginal bleeding). Vomiting and diarrhea were not significantly different between the groups. Nausea was the most frequent side effect noted in 19.7% of women in the diclofenac + misoprostol group, as compared to only 4.4% of those pretreated solely with diclofenac.
Conclusion
In cases of intrauterine contraceptive devices (IUCDs) insertion, there was between different doses of misoprostol (400 vs. 200) regarding degree of pain, success of insertion, women’s satisfaction or pharmacological side effects However needing for analgesia was significantly lower and adverse effects as abdominal cramping and shivering were significantly higher in women received higher doses of misoprostol.
Disclosure Statement
The authors declare that there is no conflict of interest associated with this manuscript.
Funding
This study received no financial support.
References
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American College of Obstetricians and Gynecologists (ACOG): Long-Acting reversible contraception: Implants And intrauterine devices. Practice Bulletin No. 186. Obstet Gynecol. 2017;130:e251–69.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123(3):585-92.
- Khalaf M, Amin AF, Sayed Z, et al. A randomized double-blind controlled trial of two different doses of self-administered vaginal misoprostol for successful copper intrauterine device insertion. Middle East Fertil Soc J. 2017;22(4):264-8.
- Bahamondes MV, Hidalgo MM, Bahamondes L, et al. Ease of insertion and clinical performance of the levonorgestrel-releasing intrauterine system in nulligravidas. Contraception. 2011;84(5):e11-6.
- Gonie A, Worku C, Assefa T, et al. Acceptability and factors associated with post-partum IUCD use among women who gave birth at bale zone health facilities, Southeast-Ethiopia. Contracept Reprod Med. 2018;3(1):1-8.
- Abbas AM, Abd Ellah NH, Hosny MA, et al. Self-administrated vaginal 2% lidocaine in-situ gel for pain relief during copper intrauterine device insertion in women with previous caesarean delivery only: a randomised, double-blind placebo-controlled trial. Eur J Contracept Reprod Health Care. 2021;26(2):132-8.
- Maged AM, Youssef G, Eldaly A, et al. Benefits of vaginal misoprostol prior to IUD insertion in women with previous caesarean delivery: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2018;23(1):32-7.
- Edelman AB, Schaefer E, Olson A, et al. Effects of prophylactic misoprostol administration prior to intrauterine device insertion in nulliparous women. Contraception. 2011;84(3):234-9.
- Espey E, Singh RH, Leeman L, et al. Misoprostol for intrauterine device insertion in nulliparous women: a randomized controlled trial. Am J Obstet Gynecol. 2014;210(3):208-e1.
- Mansy AA. Does sublingual misoprostol reduce pain and facilitate IUD insertion in women with no previous vaginal delivery? A randomized controlled trial. Middle East Fertil Soc J. 2018;23(1):72-6.
- Dijkhuizen K, Dekkers OM, Holleboom CA, et al. Vaginal misoprostol prior to insertion of an intrauterine device: an RCT. Hum Reprod. 2011;26(2):323-9.
- Scavuzzi A, Souza AS, Costa AA, et al. Misoprostol prior to inserting an intrauterine device in nulligravidas: a randomized clinical trial. Hum Reprod. 2013;28(8):2118-25.
- Ibrahim ZM, Sayed Ahmed WA. Sublingual misoprostol prior to insertion of a T380A intrauterine device in women with no previous vaginal delivery. Eur J Contracept Reprod Health Care. 2013;18(4):300-8.
- El-Gawad A, Elshahid EN, ATIK A. Vaginal Misoprostol Prior to Intrauterine Contraceptive Device Insertion in Women Who Delivered Only By Elective Caesarean Section: Randomized Clinical Trial. Evidence Based Women's Health J. 2021;11(1):74-82.
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Author Info
Ahmed Alanwar*, Sherif Naguip, Alaa El-Ghanam and Haitham El-SabaaCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.