Research - (2025) Volume 20, Issue 1
The role of FDG PET-CT-Scan in initial staging of breast cancer in Iraqi female patients
Riyadh W. AL Esawi*, Huda Mahmood Shakir, Mundher Mudhafar and Afkar Jawad AbedReceived: 01-Jan-2025, Manuscript No. gpmp-25-157488; Editor assigned: 03-Jan-2025, Pre QC No. P-157488; Reviewed: 16-Jan-2025, QC No. Q-157488; Revised: 23-Jan-2025, Manuscript No. R-157488; Published: 31-Mar-2025
Abstract
Background: To establish the optimal treatment for a breast cancer patient, proper staging is required. High sensitivity for identifying extra-axillary lymph nodes and distant metastases is provided by 18F-Fluorodeoxyglucose Positron Emission Tomography coupled with Computed Tomography (FDG-PET/CT). Our study is to evaluate the role of FDG-PET/CT in breast cancer staging and follow-up after treatment in Iraqi women.
Aims of the study: This study aims to evaluate the usefulness of Positron Emission Tomography-Computed Tomography (PET-CT) in breast cancer patients for the following: Initial breast cancer staging in Iraqi women in comparison with clinical staging and Follow up PET-CT scan to evaluate treatment response.
Patients and methods: A total of 100 female patients, ranging in age from 22 to 80 years, were included in this retrospective analysis. For initial staging with fluorine-18 fluorodeoxyglucose-positron emission tomography-computed tomography, all patients who had been diagnosed with breast cancer through biopsy were referred for a PETCT scan. Out of 100 patients, 15 had undergone a second FDG-PET-CT scan six months after treatment to determine the response. The study was carried out between January 2022 and December 2022, and all patients had undergone a tru-cut biopsy of the lump and clinical staging using a physical, histopathological, and imaging exam, as well as PET staging. The FDG PET/CT scan was carried out at the oncology and nuclear medicine center of the Amir Al-Momineen specialized hospital in Najaf. The results were reported by experienced specialists in nuclear medicine.
Results: The median patient age 50 (range: 22-80) years, the majority of the patients aged older than 40 years. The breast lump was the main complaint. 51 percent of lesions are located on the left side, where the upper-outer quadrant was the most common location of lesions. IDC (invasive ductal carcinoma) was the commonest type reported in 79% of cases followed by ILC (invasive lobular carcinoma) in 14%. The mean Standardized Uptake Values was significantly increased with advancing stage, (P. value<0.05). Receiver Operating Characteristic (ROC) curve analysis was performed using the SUV values as a scale parameter for prediction. This analysis revealed that PET scan with an optimal SUV cutoff point of 3.8 had good performance produced an area under the curve of 0.843, sensitivity of 85.7%, specificity of 76.3%, accuracy of 81%, Positive Predictive Value (PPV) of 78.3% and a Negative Predictive Value (NPV) of 84.2% which reflect good performance and validity.
Conclusions: PET/CT scan is an indispensable imaging technique had good performance and diagnostic accuracy for initial staging and follow-up of patients with breast cancer. It was effective in the evaluation of response to treatment and outcome of the patients.
Keywords
Breast cancer; FDG; PET CT SCAN
Introduction
Breast cancer is currently the most commonly diagnosed cancer type, accounting for 1 in 8 cancer diagnoses worldwide. There were approximately 2.3 million new cases of breast cancer worldwide in 2020, with approximately 685,000 deaths, with significant geographical variation observed between countries and world regions [1]. Survival rates vary from 27% to 99% depending on the stage of the disease, histological and molecular subtypes, and genetic profile of the breast cancer [2]. To improve therapeutic choices and clinical outcomes in newly diagnosed breast cancer patients, it is essential to precisely determine the degree of regional and distant disease. The National Comprehensive Cancer Network and the European Society for Medical Oncology guidelines state that using 18F-FDG PET/CT is not routinely indicated for the initial staging of breast cancer in patients whose clinical stage is between I and operable III unless there is a suspicion of metastatic disease [3,4]. According to a guideline from the European Society for Medical Oncology, PET/CT can be used instead of a CT scan and a bone scan, but not both [5]. In patients with advanced breast cancer whose imaging is suspicious but not diagnostic of metastasis, the National Institute for Health and Clinical Excellence guideline suggests PET/CT mainly for the diagnosis of metastatic disease [6]. Although the National Oncologic PET Registry, which supports the decision for PET coverage, has a limited database of breast cancer, the Centers for Medicare and Medicaid Services in the United States reimburses 18F-FDG PET in breast cancer staging for distant metastases, with the exception of axillary lymph nodes [7]. According to a recently published systematic review, the currently available evidence reveals that 18F-FDG PET/CT has greater diagnostic value when compared to other staging modalities for the detection of local and distant metastases in newly diagnosed breast cancer [8]. As a result of a growing body of information which is supported by new findings on 18F-FDG PET/CT, the initial staging and therapeutic management of breast cancer have significantly changed in recent decades [9,10]. This body of research indicates that the yield from PET/CT is significant not just for high-risk patients (those with locally progressed or inflammatory breast cancer), but also for intermediate-risk individuals with clinical stage IIB illness or higher [11]. Even more studies have suggested that 18F-FDG PET/CT may have a significant impact on early breast cancer [12,13].
Patients and Methods
A total of 100 female patients, ranging in age from 22 to 80 years, were included in this retrospective analysis. For initial staging with fluorine-18 fluorodeoxyglucose-positron emission tomography-computed tomography, all patients who had been diagnosed with breast cancer through biopsy were referred for a PETCT scan. Out of 100 patients, 15 had undergone a second FDG-PET-CT scan six months after treatment to determine the response. The study was carried out between January 2022 and December 2022, and all patients had undergone a true-cut biopsy of the lump. The FDG PET/CT scan was carried out at the oncology and nuclear medicine center of the Amir Al-Momineen specialized hospital in Najaf. The results were reported by an experienced specialist's in nuclear medicine.
Inclusion criteria: Female patients with a histopathologically confirmed diagnosis of breast cancer who did not received any treatment, with no age predilection.
Exclusion criteria: Patients with high blood glucose concentrations >200 mg/dl to avoid FDG misinterpretation, high serum creatinine concentrations >1.3 mg/dl, patients underwent surgery or received radiotherapy as a line of treatment before PET.
Methods: All patients had biopsy-proven breast cancer and clinical staging using a physical, histopathological, and imaging exam according to the AJCC staging system, as well as PET staging.
All patients had ultrasounds reports, MRI and CT scan reports, then the clinical staging was done according to AJCC staging system using the following tables (Tab. 1. and Tab. 2.):
| Tumours | T0/Tis | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Tumour size | T0: No primary tumour Tis: Tumour only in brest ducts or lobules | ≤ 2cm | >2- ≤ 5cm | >5cm | Tumour of any size with extension to chest wall/ skin ulceration or skin nodules) |
| Nodes | N0 | N1 | N2 | N3 | |
| N0 lymph node metastases | Metastases in 1-3 axilary lymph nodes | Metastases in 4-9 axilary lymph nodes | Metastases in infra- or supracalvicular lymph nodes or in ≥ 10 axilary lymph nodes | ||
| Metastasis | M0 | M1 | |||
| No evidence of cancer metastasis | Cancer found in other areas of body |
Tab. 1. Ultrasounds reports.
| AJCC | TNM | NCCN | ||
|---|---|---|---|---|
| Stage I | T1 | N0 | M0 | Primary operable breast cancer |
| Stage IIA | T0 | N1 | M0 | |
| T1 | N1 | M0 | ||
| T2 | N0 | M0 | ||
| Stage IIB | T2 | N1 | M0 | |
| T3 | N0 | M0 | ||
| Stage IIIA | T3 | N1 | M0 | Locally advanced breast cancer |
| T0 | N2 | M0 | ||
| T1 | N2 | M0 | ||
| T2 | N2 | M0 | ||
| T3 | N2 | M0 | ||
| Stage IIIB | T4 | N0 | M0 | |
| T4 | N1 | M0 | ||
| T4 | N2 | M0 | ||
| Stage IIIC | Any T | N3 | M0 | Metastatic disease |
| Stage IV | Any T | Any N | M1 |
Tab. 2. MRI and CT scan reports.
The patients were examined in a single center using a single protocol and PET-CT machines (GE Discovery IQ 3-ring PET CT system) using standard protocol, imaging from vertex to mid-thigh, caudocranially PET acquisition was acquired in 2-3 minutes per bed post 65 minutes uptake time after injection (I.V.) of fluorine-18 fluorodeoxyglucose, subsequently, with and without attenuation correction and the Q-clear algorithm, axial, coronal, and sagittal Positron Emission Tomography (PET) images were analyzed and corresponding CT images without oral or IV contrast studies conducted with an Optima 540 16-slice CT, reassembled, and merged with the Positron Emission Tomography (PET) images.
Group B: 15 out of 100 patients referred for follow-up after therapy eight patients underwent surgery, chemoradiotherapy, and radiotherapy; seven patients received chemotherapy, radiotherapy, and hormonal therapy.
Imaging: All data are acquired with a combined PET/CT in-line system (GE Discovery IQ 3-ring PET/CT system). This customized system combines a positron emission tomography scanner with a multi-section, sixteen-helical computed tomography scanner, allowing for simultaneous collection of co-registered computed tomography and positron emission tomography pictures.
Interpretation of images: We used the eighth Edition of the TNM staging methodology for breast cancer developed by AJCC to analyze all CT, PET and fused PET-CT images in aspects of initial cancer mass, lymph node involvement, and distance metastases. Qualified nuclear medicine doctors reviewed each PET/CT study. Both CT and fused PET/CT images were used to evaluate the breast cancers as well as the nodal and distant metastases. Lesions were classified as pathological if their enhanced glucose uptake was greater than that of the surrounding tissue, the chest mediastinal blood, background activity in the rest of the body, or their Standard Glucose Uptake Value (SUV) was greater than 2.5. The SUV was identified by manually sketching a Region Of Interest (ROI) of 5–10 mm over the lesion's most active area. For the ipsilateral and contralateral axillary, internal mammary, hilar, mediastinal, and pelvi-abdominal lymph node groups, the nodal tumor infiltration should be assessed as positive or negative. Any lymph node in a CT scan with a necrotic mass or a short-axis diameter of more than 10 mm was labeled as malignant, while any lymph node with a fatty hilum, regardless of size, was categorized as benign. Even though a lymph node's short axis diameter was less than 1 cm, metastatic spread was still presumed to have occurred if its glucose concentration was elevated during PET scans. Even if non-FDG-avid lymph nodes measured greater than 1 cm in short-axis diameter, they were still regarded as benign (negative for metastatic dissemination) in PET imaging. The presence of tumor infiltration was evaluated for each of the following areas: lung, visceral organs (liver, spleen, and adrenal glands), brain, and bone. Each of these areas was examined for the presence of distant metastases. If FDG uptake exceeds the mediastinal blood pool in patients with 5-mm lung nodules, they should be considered positive. It is impossible to rule out metastatic lung deposits if the nodule is less than 5 mm. Lesions that have a higher uptake than the liver or spleen are considered positive hepatic or splenic lesions. When it comes to lesions on the adrenal glands, benign lesions are defined as those with a density of less than 10 HU; if the density is higher than 10 HU, the SUVmax of the lesion should be assessed. Then, if the SUV maximum was less than 3.1 or greater than 3.1, they were categorized as benign and malignant, respectively. Patients with localized bone marrow lesions and enhanced FDG uptake were taken into consideration if they tested positive for osseous deposits.
Results
A total of 100 female patients were recruited in the study, 77% of them were resident in Najaf and the remaining patients were resident in Karbala, Babil and Baghdad in a rate of 16%, 5% and 2%, respectively. A mean age of 50.1 ± 11 (range: 22–80) years. Additionally, majority of the patients aged older than 40 years where only 18 patients aged 40 years or younger, 35 patients aged 41–50 years, 30 patients aged 51-60 years and 17 women were older than 60 years. Housewives contributed for 87% of the studied group, and 15% had positive family history of breast cancer (Tab. 3.).
| Variable | No. | % | |
|---|---|---|---|
| Age (year) | ≤ 40 | 18 | 18 |
| 41 - 50 | 35 | 35 | |
| 51 - 60 | 30 | 30 | |
| > 60 | 17 | 17 | |
| Mean ± (SD) | 50.1 ± 11 | - | |
| Range | 22 - 80 | - | |
| Occupation | Housewife | 87 | 87 |
| Employed | 13 | 13 | |
| Family history of breast cancer | Yes | 15 | 15 |
| No | 95 | 95 | |
| SD: Standard Deviation of mean | |||
Tab. 3. Demographic characteristics of the studied group.
All patients presented with breast lump, 45 women with pain and or tenderness at the affected breast and only 4 women presented with Nipple discharge (Fig. 1.).
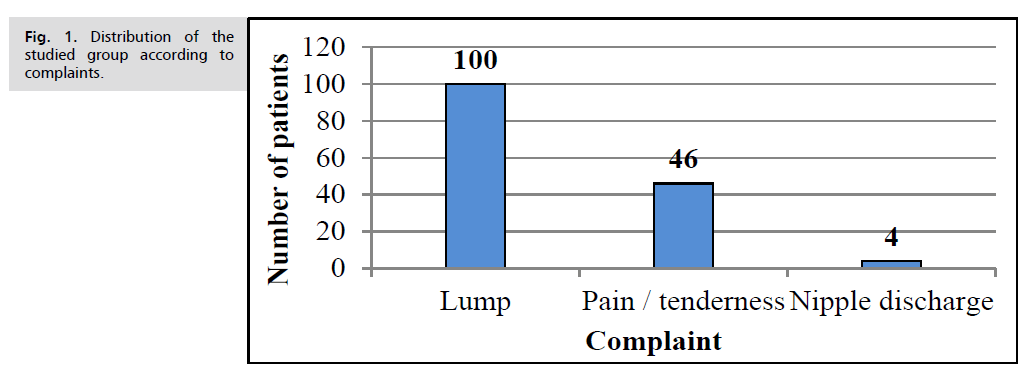
Fig. 1. Distribution of the studied group according to complaints.
Secondary lesions in chest wall reported in 25% of cases followed by MSK (20%), mediastinum 14%, lung 10%, liver 7%, head and neck 3%, pelvis 1% and Suprarenal gland 1%. LAP other than axillary LNs reported in 42% of cases (Tab. 4.).
| Characteristic | No. | % | |
|---|---|---|---|
| Laterality | Left | 51 | 51 |
| Right | 47 | 47 | |
| Bilateral | 2 | 2 | |
| Location | Upper-outer quadrant | 40 | 40 |
| Upper-inner quadrant | 7 | 7 | |
| Lower-outer quadrant | 12 | 12 | |
| Lower-inner quadrant | 12 | 12 | |
| Retro-areolar | 27 | 27 | |
| Other | 2 | 2 | |
| Number of lesions | Single | 92 | 92 |
| Multiple | 8 | 8 | |
| Outline | Irregular Spiculated | 100 | 100 |
Tab. 4. Characteristics of breast lesions of the studied group (N=100).
The histopathological types of breast cancer of the studied group where IDC was the commonest type reported in 79% of cases, ILC in 14%, Mixed IDC & ILC in 3% and other types were Stromal sarcoma (2), DCIS (1), Adenocarcinoma (1).
Initial PET staging of the studied group revealed that 8% of patients at stage IA, 12% stage IIA, 15% stage IIB, 15% stage IIIA, 13% stage IIIB and 37% at stage IV is shown in Tab. 5.-Tab. 9. and Fig. 2.
| Site | No. | % |
|---|---|---|
| Chest wall | 25 | 20.3 |
| MSK | 20 | 16.3 |
| Mediastinum | 14 | 11.4 |
| Lung | 10 | 8.1 |
| Liver | 7 | 5.7 |
| Head and Neck | 3 | 2.4 |
| Pelvis | 1 | 0.8 |
| Suprarenal gland | 1 | 0.8 |
| LAP other than axillary | 42 | 34.1 |
| Total secondary lesions and LAP | 123 | 100 |
Tab. 5. Distribution of secondary lesions and LAP.
| Lesion | Total number* | Median Size (cm) | Largest lesion (cm) | Smallest lesion (cm) |
|---|---|---|---|---|
| Primary breast lesion | 107 | 2.5 × 1.7 × 1.5 | 7.0 × 5.5 × 5.3 | 0.5 × 0.4 × 0.4 |
| Axillary lymph nodes | 223 | 1.7 × 1.1 × 1.0 | 3.2 × 2.1 × 1.9 | 1.1 × 0.5 × 0.4 |
| Secondary / liver lesions | 4 | 2.7 × 1.8 × 1.3 | 8.4 × 6.0 × 5.2 | 1.5 × 1.3 × 1.1 |
| *Some patients had more than one lesion and multiple axillary LAP | ||||
Tab. 6. Size of primary and secondary lesions of the studied group (N =100).
| TNM staging | No. | % |
|---|---|---|
| T1N0M0 | 11 | 11 |
| T1N1M0 | 9 | 9 |
| T1N2M0 | 4 | 4 |
| T2N0M0 | 17 | 17 |
| T2N1M0 | 15 | 15 |
| T2N2M0 | 20 | 20 |
| T2N2M1 | 6 | 6 |
| T2N3M1 | 7 | 7 |
| T3N2M0 | 4 | 4 |
| T4N2M0 | 4 | 4 |
| T4N3M0 | 3 | 3 |
| Total | 100 | 100 |
Tab. 7. Clinical staging according to TNM staging of breast lesions of the studied group.
| Grouped Staging | No. | % |
|---|---|---|
| IA | 7 | 7 |
| IB | 4 | 4 |
| IIA | 25 | 25 |
| IIB | 16 | 16 |
| IIIA | 28 | 28 |
| IIIB | 9 | 9 |
| IV | 11 | 11 |
| Total | 100 | 100 |
Tab. 8. Clinical grouped staging of breast cancer.
| Grouped Staging | No. | % |
|---|---|---|
| IA | 8 | 8 |
| IIA | 12 | 12 |
| IIB | 15 | 15 |
| IIIA | 15 | 15 |
| IIIB | 13 | 13 |
| IV | 37 | 37 |
| Total | 100 | 100 |
Tab. 9. Initial PET staging of breast cancer.
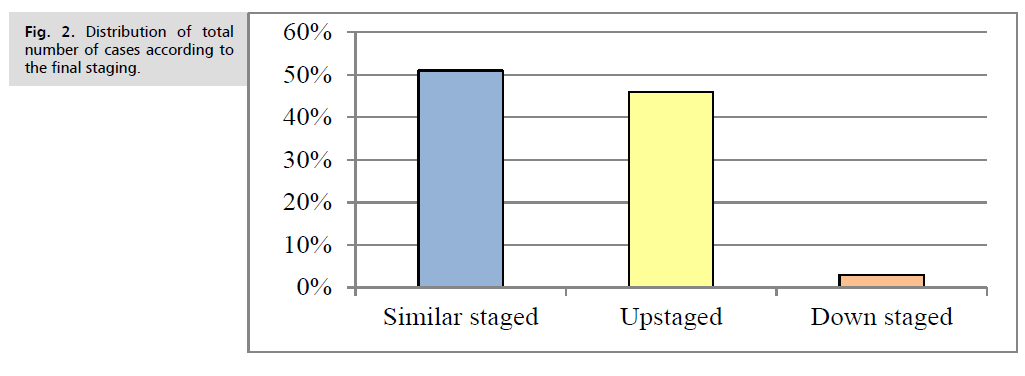
Fig. 2. Distribution of total number of cases according to the final staging.
Descriptive Statistics of SUV of primary and secondary lesions and axillary lymph nodes are summarized in Tab. 10.
| PET staging | Total | Upstaged | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial stage | IA | IIA | IIB | IIIA | IIIB | IV | No. | % | ||
| IA | 6 | 1 | 7 | 1 | 14.30% | |||||
| IB | 1 | 1 | 2 | 4 | 3 | 75.00% | ||||
| IIA | 2 | 11 | 1 | 2 | 6 | 3 | 25 | 12 | 48.00% | |
| IIB | 12 | 2 | 2 | 16 | 4 | 25.00% | ||||
| IIIA | 9 | 5 | 14 | 28 | 19 | 67.90% | ||||
| IIIB | 2 | 7 | 9 | 7 | 77.80% | |||||
| IV | 11 | 11 | 0 | 0.00% | ||||||
| Total | 8 | 12 | 15 | 15 | 13 | 37 | 100 | 46 | 46.00% | |
| Upstaged | ||||||||||
| Down staged | ||||||||||
| Same staging | ||||||||||
Tab. 10. Distribution of upstaged cases following PET /CT.
No significant difference had been found between the three SUV values of the primary, secondary or axillary lymph nodes, (P. value >0.05).
A direct (positive) correlation was found between higher SUV value and each of advanced primary staging, spiculated outlined lesion and advanced PET staging, (P. value<0.05). No significant correlation was found between SUV values and each of histopathology types and location of breast cancer, (P. value >0.05).
Furthermore, correlation between PET staging of breast cancer and other parameters was assessed and demonstrated in Tab. 11.
| Statistics | SUV Primary lesion | SUV Secondary lesion | SUV Axillary LN | P. value |
|---|---|---|---|---|
| Mean | 5.36 | 6.67 | 6.14 | 0.539 ns |
| Median | 5.5 | 6.35 | 6 | |
| SD | 3.91 | 3.97 | 3.9 | |
| Minimum | 1.5 | 1.9 | 1.5 | |
| Maximum | 16.7 | 21 | 18 | |
| SD: Standard Deviation, ns: not significant | ||||
Tab. 11. Descriptive statistics of SUV of primary and secondary lesions.
Where a statistically significant correlation was found between PET staging and each of Initial advanced staging and outline. Moreover, the mean SUV value was compared across the grouped staging of primary lesions, advancing stage, (P. value<0.05) (Fig. 3.).
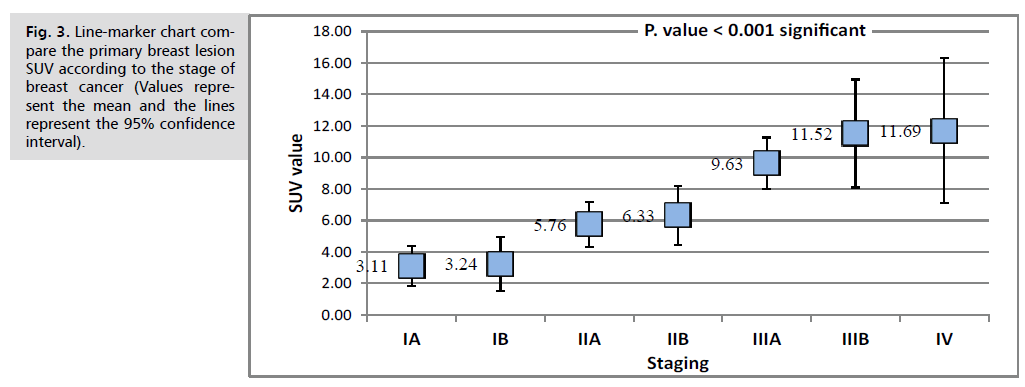
Fig. 3. Line-marker chart compare the primary breast lesion SUV according to the stage of breast cancer (Values represent the mean and the lines represent the 95% confidence interval).
To assess the validity of PET scan in prediction of advanced stages of breast cancer, Receiver Operating Characteristic (ROC) curve analysis was performed using the SUV values as a scale parameter for prediction. This analysis revealed that PET scan with an optimal SUV cut-off point of 3.8 had good performance produced an area under the curve of 0.843, sensitivity of 85.7%, specificity of 76.3%, accuracy of 81%, Positive Predictive Value (PPV) of 78.3% and a Negative Predictive Value (NPV) of 84.2% which reflect good performance and validity Tab. 12. and Tab. 13. and Fig. 4.
| Variable | Correlations vs. SUV primary lesion | |
|---|---|---|
| R | P. value | |
| Histopathology | 0.054 | 0.592 ns |
| Initial clinical staging (advanced) | 0.492 | <0.001 |
| PET staging | 0.422 | <0.001 sig |
| Location | 0.108 | 0.283 ns |
| Outline (spiculated) | 0.208 | 0.037 sig |
Tab. 12. Correlation between SUV of primary lesion and other parameters.
| Variable | Correlations vs. PET staging | |
|---|---|---|
| R | P. value | |
| Histopathology | 0.117 | 0.183 ns |
| Clinical Staging (advanced) | 0.649 | <0.001 sig |
| Location | 0.13 | 0.113 ns |
| Outline (Spiculated) | 0.32 | <0.001 sig |
Tab. 13. Correlation between PET staging of breast cancer and other parameters.
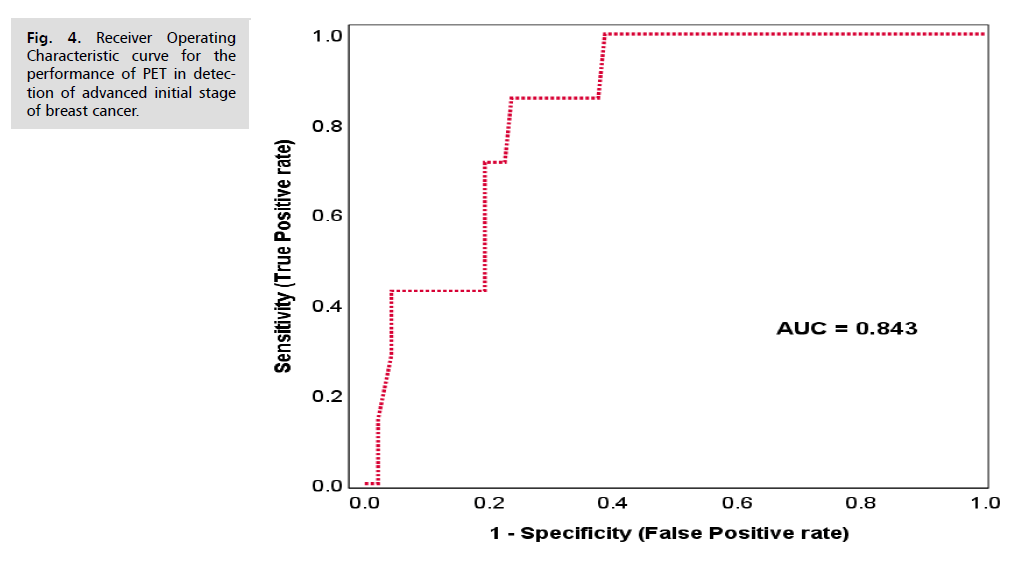
Fig. 4. Receiver Operating Characteristic curve for the performance of PET in detection of advanced initial stage of breast cancer.
According to the PET scan staging at initial and post treatment, a significant change was found in staging before and after treatment, (P. value< 0.05), after treatment, 4 patients became at stage zero, 4 patients at stage IA, 4 patients stage IIA and 3 patients at stage IV (Tab. 14.).
| Histopathology | Number of cases | Mean | SD | Range | |
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| IDC | 79 | 5.8 | 3.71 | 1.5 | 16.7 |
| ILC | 14 | 2.97 | 2.06 | 1.53 | 7.43 |
| Mixed IDC & ILC | 3 | 3.93 | 4.16 | 1.53 | 8.73 |
| Stromal sarcoma | 2 | 10.28 | 0.21 | 10.13 | 10.43 |
| DCIS | 1 | 1.53 | 0 | 1.53 | 1.53 |
| Adenocarcinoma | 1 | 2.53 | 0 | 2.53 | 2.53 |
| P. value=0.386, no significant difference | |||||
Tab. 14. Descriptive statistics of SUV of primary and secondary lesions.
Down staging reported in 12 cases (75%), while 3 cases (25%) staged the same as initial staging, (P. value< 0.05) (Tab. 15.).
| Parameter | Value |
|---|---|
| Optimal cutoff point | 3.8 |
| AUC | 0.843 |
| Sensitivity | 85.70% |
| Specificity | 76.30% |
| Accuracy | 81.00% |
| PPV | 78.30% |
| NPV | 84.20% |
Tab. 15. Validity parameters of PET-SUV of primary lesion for the detection of initial advanced stage of breast cancer.
Additionally, the outcomes of the 15 patients who were followed up and assessed revealed Complete/excellent response in 8 cases, Partial Response in 4 cases and Recurrence/progression in 3 cases. From other point of view, the mean SUVmax value was significantly lower in cases with complete/excellent response (the mean SUVmax=1.9 ± 1.8), increased in those with partial response (the mean SUVmax=4.9 ± 0.6) and much higher in those with recurrence/progression, (the mean SUVmax=9.9 ± 2.4), (P. value<0.001), as shown in Tab. 16.-Tab. 18. and Fig. 5.
| Number and percentages of cases | ||||
|---|---|---|---|---|
| Before Treatment | After Treatment | |||
| PET staging | No. | % | No. | % |
| Stage 0 | 0 | 26.7 | 4 | 0 |
| Stage IA | 2 | 6.7 | 4 | 13.3 |
| Stage IIA | 2 | 20 | 4 | 13.3 |
| Stage IIB | 1 | 0 | 0 | 6.7 |
| Stage IIIA | 1 | 0 | 0 | 6.7 |
| Stage IIIB | 3 | 0 | 0 | 20 |
| Stage IV | 6 | 46.7 | 3 | 40 |
| Total | 15 | 100 | 15 | 100 |
| P. value=0.040 significant (Fisher’s exact test) | ||||
Tab. 16. PET scan initial and post treatment staging.
| Initial PET staging | |||||||
|---|---|---|---|---|---|---|---|
| Post treatment PET staging | Stage IA | Stage IIA | Stage IIB | Stage IIIA | Stage IIIB | Stage IV | Total (Post) |
| Stage 0 | 2 | 2 | 4 | ||||
| Stage IA | 1 | 1 | 2 | 4 | |||
| Stage IIA | 1 | 3 | 4 | ||||
| Stage IV | 3 | 3 | |||||
| Total (Initial) | 2 | 2 | 1 | 1 | 3 | 6 | 15 |
| Total down-staged | 12 (75%) | P. value=0.030 significant (Fisher’s exact test) | |||||
| Total Same staged | 3 (25%) | ||||||
Tab. 17. Cross-tabulation for initial and post treatment PET scan staging of breast cancer.
| Outcome | No. of cases | SUV Primary | P. value | |
|---|---|---|---|---|
| Mean | SD | |||
| Complete/excellent response | 8 | 1.9 | 1.8 | <0.001 ANOVA test |
| Partial Response | 4 | 4.9 | 0.6 | |
| Recurrence/progression | 3 | 9.9 | 2.4 | |
Tab. 18. Comparison of SUV of primary lesion after treatment according to stage and outcome of 15 followed up patients.
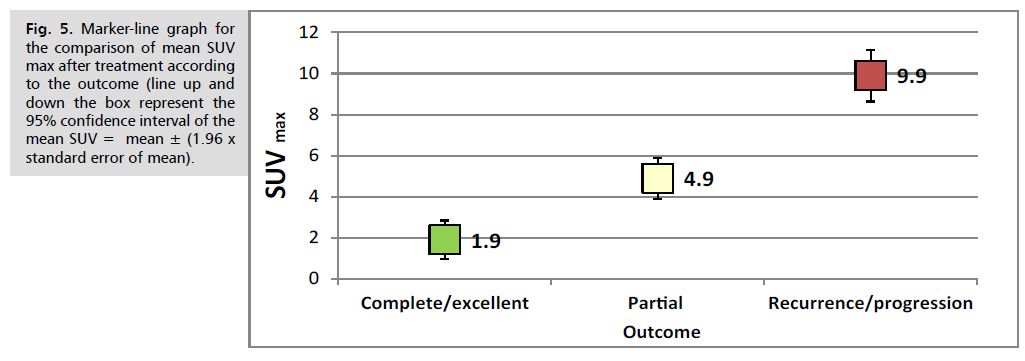
Fig. 5. Marker-line graph for the comparison of mean SUV max after treatment according to the outcome (line up and down the box represent the 95% confidence interval of the mean SUV = mean ± (1.96 x standard error of mean).
Discussion
Breast cancer remains one of the commonest cancers worldwide. It contributes for almost 25% of all cancer cases. In Iraq, currently, breast cancer ranks number one among all incident cancers [14,15].
Accurate staging is crucial in determining the appropriate course of treatment and predicting patient outcomes. Combining Computed Tomography (CT) with 18F-Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) is a powerful imaging modality for both initial staging and follow-up of breast cancer [16].
The FDG PET/CT scan is particularly useful in identifying distant metastases, such as bone, liver, and lung involvement, which may be missed by other imaging methods. Compared to traditional imaging modalities, the sensitivity and specificity of FDG PET/CT in detecting breast cancer recurrence are higher, especially in patients with suspicious clinical findings or elevated tumor markers [17,18].
Although FDG PET/CT is valuable in breast cancer management, it has some limitations, such as lower sensitivity for small lesions and false-positive results from inflammation or infection. Furthermore, it may not be cost-effective for every patient, necessitating the use of this imaging modality on a case-by-case basis [19]. Therefore, studies are still conducted for more precise evaluation of the role of FDG PET CT scan in initial staging and follow up of breast cancer patients and there still a need for further studies particularly in our country, hence the current study tried to fill part of the gap in scientific literatures relevant to this imaging modality.
The Present study aimed to assess the role of FDG PET CT scan in initial staging and follow up of Iraqi women with different stages of breast cancer. The study included 100 Iraqi women aged between 22-80 years with a mean of 50.1 ± 11. The age distribution of the studied group consistent with the epidemiological picture of breast cancer, where its incidence increases with advancing age. Among the studied group, majority were older than 40 years, which is also reported in previous Iraqi studies [20,21].
In the present study the characteristics of breast lesions of the studied group with regard to the complaints of the patients, where all presented with breast lump and it was painful in 45% of cases while nipple discharge reported in only 4%, these findings were not unexpected and in line with the clinical picture of breast cancer. Mutar MT, et al. [21] found that more than 70% of cases presented with breast lump and almost 19% had pain. Left side lesions were relatively frequent among our patients and only two cases had bilateral lesions, this is consistent with that reported in earlier studies where bilateral lesions are less commonly reported in breast cancer cases. On the other hand, upper quadrant lesions were more frequent and contributed for 40% of all lesions , most of lesions were single and these findings agreed that reported in literatures [22].
Metastasis of breast cancer and invasion of adjacent organs and lymph nodes are not uncommon where secondary lesions in chest wall are frequently reported, musculoskeletal structures, mediastinum lung and liver and to less extent, head and neck and pelvis , however, in cancer cases in general , metastasis cannot easily predicted and many organs may be affected [23].
According to the histopathological types of breast cancer of the studied group, IDC was the commonest type reported in 79% of cases, many studies and literatures supported this finding due to the fact that IDC is the most common reported type [24].
The SUV of primary and secondary lesions and axillary lymph nodes were not significantly difference (P. value >0.05). However, when we assessed the correlation of SUV with other parameters, we found a significant direct correlation between higher SUV values and each of advanced primary staging, spiculated outlined lesion and advanced PET staging, (P. value<0.05) and no significant correlation between SUV values and each of histopathology types and location of breast cancer, (P. value >0.05). Furthermore, a statistically significant correlation was found between PET staging and each of Initial advanced staging and outline. These findings are reported by AbdElaal AA, et al. [25]. These findings reflect the beneficial and valuable role of PET scan in staging of breast cancer. This fact approved by the analysis that performed with Receiver Operating Characteristic (ROC) curve analysis which revealed that PET scan with an optimal SUV cutoff point of 3.8 had good performance and produced an area under the curve of 0.843, giving a sensitivity of 85.7%, specificity of 76.3%, accuracy of 81%, Positive Predictive Value (PPV) of 78.3% and a Negative Predictive Value (NPV) of 84.2% which reflect good performance and validity. From other point of view, SUV values had similar trend with regard to the higher values in advancing stages when we assessed 15 patients after treatment and during the follow up. It had been found that higher SUV values associated with advancing stages and poor prognosis where the highest SUV value reported in cases with stage IV and in those with recurrence or progression compared to those with lower stages and good outcomes. Almost similar findings also reported in previous studies; in a multicenter study conducted by Hyland CJ, et al. [26]. FDG PET scan was evaluated as an initial staging modality in breast cancer, authors concluded that PET/CT was good for earlier evaluation and initiation of treatment and can easily differentiate metastatic staging and improve practice. Han S, et al. [16] assessed the effectiveness of 18F-FDG, PET/CT and PET/MRI in staging and management as a modality for initial staging in breast cancer and concluded that these modalities lead to a significant improvement in the staging and treatment of newly diagnosed cases with breast cancer and they can be considered for routine clinical use.
Botsikas D, et al. [27] reported the previous findings where they documented that PET/CT was comparable to PET/MRI with good performance per patient, however, for all lesions together, PET/MRI remains superior to PET/CT (In the lesion-per-lesion analysis, the sensitivity of PET/MR and PET/CT for bone metastases, other metastases, axillary and internal mammary nodes, contralateral tumors and all lesions together was 0.924 and 0.6923 (p=0.0034), 0.923 and 0.923 (p=1), 0.854 and 0.812 (p=0.157), 0.9 and 0.9 (p=1), and 0.25 (p=0.083), and 0.89 and 0.77 (p=0.0013) respectively. The corresponding specificity was 0.953 and 1 (p=0.0081), 1 and 1 (p=1), 0.893 and 0.92 (p=0.257), 1 and 1 (p=1), 0.987 and 0.99 (p=1) and 0.96 and 0.98 (p=0.0075) respectively).
From other point of view, Hildebrandt MG, et al. [28] stated in their recent study in 2022 that a strong evidence was found suggesting the superiority of PET/CT compared to conventional imaging techniques in evaluation and follow up of response to treatment in metastatic breast cancers. Nonetheless, in breast cancers types with low glucose metabolism like lobular subtypes of metastatic breast cancer, PET/scan could not be beneficial for monitoring and follow up. Also, they concluded that PET/CT was highly sensitive in prediction of progressing and regressive breast cancer.
Bruckmann NM, et al. [29] compared the diagnostic accuracy of PET/CT with other modalities including PET/MRI, MRI, CT and bone scintigraphy for detection of bone metastasis during the initial staging of breast cancer, they found that PET/MRI was better than CT or bone scintigraphy and they are both had limited sensitivity in detection of metastatic lesions to the bones [29].
Another recent study in 2022, conducted by Krajnc D, et al. [30] concluded that PET/CT had higher diagnostic accuracy and good performance in prediction of triple negative molecular subtypes of breast cancer.
Groheux D, in his study reported the previous evidence about the beneficial role of PET/CT for primary staging of breast cancer and interestingly, Groheux D suggested that PET/CT is effective even when the tumor markers are within the normal levels [18].
Limitations of the study included the shortage in time leads to difficulty in follow up of larger group, however, the results that we obtained for the 15 patients were highly significant and could be conclusive enough to give evidence about the role of PET/CT in follow up of patients. Nonetheless, further studies with larger sample size and longer duration are still needed for better assessment.
Conclusion
PET/CT scan is an indispensable imaging technique had good performance and diagnostic accuracy for initial staging and follow-up of patients with breast cancer. It was effective in the evaluation of response to treatment and outcome of the patients.
References
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15-23.
- https://www.qeios.com/read/W3JPUC
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020;18(4):452-478.
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220.
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623-1649.
- Mroz EA, Rocco JW. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor-outcome classes of head and neck squamous cell carcinoma. Oral Oncol. 2013;49(3):211-215.
- https://www.cms.gov/medicare-coverage-database/view/ncacal-decision memo.aspx?proposed=N&NCAId=263
- Caresia Aroztegui AP, García Vicente AM, Alvarez Ruiz S, et al. 18F-FDG PET/CT in breast cancer: Evidence based recommendations in initial staging. Tumor Biol. 2017;39(10):1010428317728285.
- Chandra P, Ravichander SK, Babu SM, et al. Evaluation of diagnostic accuracy and impact of preoperative positron emission tomography/computed tomography in the management of early operable breast cancers. Ind J Nucl Med. 2020;35(1):40-47.
- Akepati NK, Abubakar ZA, Bikkina P. Role of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography scan in primary staging of breast cancer compared to conventional staging. Ind J Nucl Med. 2018;33(3):190-193.
- Groheux D, Cochet A, Humbert O, et al. 18F-FDG PET/CT for staging and restaging of breast cancer. Ind J Nucl Med. 2016;57(Supplement 1):17S-26S.
- Evangelista L, Cervino AR, Michieletto S, et al. Diagnostic and prognostic impact of fluorine-18-fluorodeoxyglucose PET/CT in preoperative and postoperative setting of breast cancer patients. Nucl Med Commun. 2017;38(6):537-545.
- Gunalp B, Ince S, Karacalioglu AO, et al. Clinical impact of 18F-FDG PET/CT on initial staging and therapy planning for breast cancer. Exp Ther Med. 2012;4(4):693-698.
- Alwan NA. Breast cancer among Iraqi women: Preliminary findings from a regional comparative breast cancer research project. J Glob Oncol. 2016;2(5):255.
- https://gco.iarc.fr/today/data/factsheets/populations/368-iraq-fact-sheets.pdf
- Han S, Choi JY. Impact of 18F-FDG PET, PET/CT, and PET/MRI on staging and management as an initial staging modality in breast cancer: A systematic review and meta-analysis. Clin Nucl Med. 2021;46(4):271-282.
- Zhang C, Liang Z, Liu W, et al. Comparison of whole-body 18F-FDG PET/CT and PET/MRI for distant metastases in patients with malignant tumors: A meta-analysis. BMC Cancer. 2023;23(1):37.
- Groheux D. FDG-PET/CT for primary staging and detection of recurrence of breast cancer. Inseminars In Nuclear Medicine 2022;52(5): 508-519.
- Groheux D, Cochet A, Humbert O, et al. 18F-FDG PET/CT for staging and restaging of breast cancer. Journal of Nuclear Medicine. 2016;57(Supplement 1):17S-26S.
- Al-Hashimi MM. Trends in breast cancer incidence in Iraq during the period 2000-2019. Asian Pac J Cancer Prev. 2021;22(12):3889.
- Mutar MT, Goyani MS, Had AM, Mahmood AS. Pattern of presentation of patients with breast cancer in Iraq in 2018: A cross-sectional study. J Glob Oncol. 2019;5:1-6.
- Yu C, Mitchell JK. Non-randomness of the anatomical distribution of tumors. Cancer Converg. 2017;1:1-6.
- Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Elsevier; 2020:14–27.
- Scholl AR, Flanagan MB. Educational Case: Invasive Ductal Carcinoma of the Breast. Acad Pathol. 2020;7:2374289519897390
- AbdElaal AA, Zaher AM, Abdelgawad MI, et al. Correlation of primary tumor metabolic parameters with clinical, histopathological and molecular characteristics in breast cancer patients at pre-operative staging FDG-PET/CT study. Egypt J Radiol Nucl Med. 2021;52:1-1.
- Hyland CJ, Varghese F, Yau C, et al. Use of 18F-FDG PET/CT as an initial staging procedure for stage II–III breast cancer: A multicenter value analysis. J Natl Compr Canc Netw. 2020;18(11):1510-1517.
- Botsikas D, Bagetakos I, Picarra M, et al. What is the diagnostic performance of 18-FDG-PET/MR compared to PET/CT for the N-and M-staging of breast cancer?. Eur Radiol. 2019;29:1787-1798.
- Hildebrandt MG, Naghavi-Behzad M, Vogsen M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin Nucl Med. 2022;52(5):520-530.
- Bruckmann NM, Kirchner J, Umutlu L, et al. Prospective comparison of the diagnostic accuracy of 18F-FDG PET/MRI, MRI, CT, and bone scintigraphy for the detection of bone metastases in the initial staging of primary breast cancer patients. Eur Radiol. 2021 Nov;31(11):8714-8724.
- Krajnc D, Papp L, Nakuz TS, et al. Breast tumor characterization using [18F] FDG-PET/CT imaging combined with data preprocessing and radiomics. Cancers. 2021;13(6):1249.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Riyadh W. AL Esawi*, Huda Mahmood Shakir, Mundher Mudhafar and Afkar Jawad AbedCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.