Research - (2024) Volume 19, Issue 4
The impact of adding estradiol to progesterone in luteal phase support in patients with in vitro fertilization
Amal Muner Mubarak*, Aseel Abdulameer Mohammed, Dalal M Jarrah and Zahraa F. AlhilliReceived: 02-Dec-2024, Manuscript No. gpmp-25-159784; Editor assigned: 03-Dec-2024, Pre QC No. P-159784; Reviewed: 12-Dec-2024, QC No. Q-159784; Revised: 19-Dec-2024, Manuscript No. R-159784; Published: 30-Dec-2024
Abstract
Background: Luteal phase support (LPS) after ICSI is a critical step, with progesterone being the standard treatment. However, the benefit of adding estradiol to this support remains controversial.
Objective: To evaluate the effect of adding oral estradiol to progesterone for luteal phase support in patients undergoing ICSI/ET.
Design: Prospective cohort study.
Setting: Fertility Center, Alsader Teaching Hospital, Najaf.
Patients and methods: A total of 180 subfertile women aged 18-35 years, undergoing ovarian stimulation and ICSI, were randomly divided into two groups for luteal phase support. The progesterone-only group (n=90) received vaginal progesterone (400 mg twice daily) and oral dydrogesterone (20 mg daily). The progesterone plus estradiol group (n=90) received oral estradiol (4 mg daily) in addition to the progesterone regimen.
Results: Demographic characteristics, stimulation parameters, and embryological data were comparable between the two groups. The clinical pregnancy rate was higher in the estradiol group (43.3%) compared to the progesterone-only group (36.7%), though this difference was not statistically significant. No significant differences were observed in ongoing pregnancy rates, early pregnancy loss, or multiple pregnancy rates between the groups. However, the implantation rate was significantly higher in the estradiol plus progesterone group (21%) compared to the progesterone-only group (13%) (P<0.001).
Conclusion: The addition of oral estradiol (4 mg) to progesterone in patients undergoing ICSI did not significantly improve overall pregnancy outcomes. However, the implantation rate was significantly higher in the estradiol plus progesterone group.
Keywords
Estradiol; Progesterone; Luteal phase; In vitro fertilization
Introduction
Luteal phase deficiency is commonly observed in individuals who undergo controlled ovarian hyperstimulation protocols in assisted reproductive technique. The potential detrimental effects of this methodology result in diminished pituitary function, pulsatile production of LH, progesterone and estrogen is seriously disrupted, and shortened luteal phase duration [1-3]. As a result, luteal phase support is essential for addressing luteal insufficiency and enhances pregnancy outcomes in IVF treatments, however there is significant debate regarding the optimal use of luteal phase support [4,5]. The most common forms of luteal phase supplementation involve the use of progesterone which is usually administered by different routes micronization techniques have enhanced the oral and vaginal bioavailability of progesterone. Among the available options the vaginal administration of micronized progesterone is generally favored by practitioners, although there is no consensus on the optimal route of use [6-8]. Other drugs that used in luteal phase support include:
Human Chorionic Gonadotropin (hCG): hCG injections of 1500 IU can be Administered every three days for a total of three doses, starting the day after oocyte retrieval during the luteal phase. However, the use of hCG is uncommon due to the higher incidence of ovarian hyperstimulation syndrome. Its use is restricted to women with hypogonadotropic hypogonadism undergoing ovulation induction, as well as patients undergoing controlled ovarian hyperstimulation (COH) who were triggered with a GnRH agonist [9-12].
GnRH-agonist: Some receptors of GnRH-a have been found in the corpus luteum, endometrium, and early embryo which seems the most probable explanation for its good results. Its use increase live birth rate and clinical pregnancy rate in both GnRH agonist and GnRH antagonist cycles [13,14].
Estradiol: The use of estradiol supplementation with progesterone to support the luteal phase in IVF/ICSI cycles has been a topic of debate. Although some studies propose that E2 supplementation could improve implantation rates [15], this claim has not been confirmed by meta-analysis findings [16].
Aim of study to evaluate the effect of adding oral estradiol treatment to progesterone in luteal phase support to patient undergoing Intra cytoplasmic sperm insemination/Embryo transfer.
Methods: This prospective cohort study was carried out in Al-Najaf city/Iraq, in Fertility Center/Al-Sader Teaching Hospital from the period between February 2022 and February 2023. The study was approved by the Ethics Committee of University of Kufa and informed consent was obtained from all participants.
A total number of participant’s 180 subfertile women undergoing IVF/ICSI cycles were included in the study. They were divided into two groups according to the drugs used for luteal phase support: first group was (90) patients who received both estrogen and progesterone as LPS (P/E2 group), and second group (90) patients who received only progesterone treatment (P group).
The inclusion Criteria were: women’s age from 18-35, BMI 19-29, presence of ovaries, their first ICSI cycle, normal basal hormonal levels; FSH and LH<10 IU/l and E2<50 pg/ml. The exclusion Criteria were: polycystic ovarian syndrome PCOS, presence of endometriosis, sever male factor and azospermia, the presence of uterine fibroids and an estradiol (E2) level above 3000 pg/ml on the day of human chorionic gonadotropin (hCG) administration., poor response i.e. ≤ 3 oocytes retrieved and/or E2 level<500 pg/ml on hCG day.
Baseline assessment: On cycle day two or three, detailed patient information was collected, including name, age, and body mass index (BMI), which is calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Duration, type, and causes of infertility. Vaginal U/S scan was performed to check the uterus, endometrial thickness, and ovaries to confirm ovarian morphology if PCOS, assessment of total antral follicular count of both ovaries and if there is any cysts.
A blood sample was taken for measurement of basal hormonal levels (FSH, LH, prolactin, TSH, testosterone, progesterone and estradiol). All men must have semen sample analysis.
Ovarian stimulation and ICSI procedure: Antagonist protocol for ovarian stimulation was implemented to all patients to decrease the bias in the result; in this protocol the treatment was started from cycle day 2 or 3 with recombinant FSH (Gonal F, Serono, Switzerland) and/or HMG (Menogon, Ferring, Germany) at a dose of 150-225 IU/ day according to the patient’s age and FSH level, the treatment continued for 9-19 days (median 14 days). During this period, the treatment cycle was monitored using transvaginal ultrasound to assess developing follicles and measure endometrial thickness starting on stimulation day 6 and then as necessary. To inhibit premature LH surge, daily GnRH antagonist cetrotide (cetrorelix, Serono, Switzerland) at a dose of 0.25 mg/day was used once the dominant follicle reached the size of 14mm and more in diameter (flexible multiple dose antagonist protocol) and continue along with gonadotropin drugs up to and including the day of hCG administration.
When the ultrasound (U/S) suggest adequate number of mature follicles (>3 follicles of ≥ 18 mm) and normal endometrial thickness (>7mm); estradiol and progesterone levels were assessed, FSH injections stopped and hCG (Pregnyl, Organon, Netherlands) was given in a dose of 10000 IU by intramuscular injection to induce oocyte maturation.
Oocyte retrieval is then done at approximately 34-36 hours after hCG injection. The procedure was done under general anesthesia by insertion a delicate needle (Gynetics/ Belgium) in to the follicle under trans-vaginal ultrasound guidance and then follicular fluid aspirated by gentle suction, the follicular fluid was passed to the laboratory for examination under microscope by the embryologist for oocyte identification.
Intracytoplasmic sperm injection ICSI was done thereafter; the sperm was aspirated and injected into the oocyte in metaphase II. Then fertilization results were assessed next day after ICSI as presence of 2PN oocytes. The fertilization rate was defined as the ratio of diploid zygotes to the total number of injected mature oocytes. Early cleavage was evaluated 48 hours after ICSI. The cleavage rate was defined as the ratio of the number of embryos to the number of diploid zygotes.
Embryo transfer was done in the 3rd day after oocyte collection, one to three selected embryo transferred to the uterus under abdominal U/S guidance.
Luteal supplementation
- Progesterone (P group): Luteal phase support was provided using oral progesterone supplementation in the form of dydrogesteron (duphastone by Abbott ) in a dose of 20 mg/day into two divided doses, and vaginal progesterone (cyclogest, Actavis, UK) 400 mg twice a day.
- Progesterone/Estradiol (P/E2 group): Similar to the progesterone group, but with the addition of oral estradiol valerate. (Estrofem, Novo Nordisk, Denmark) 4 mg/day into two divided doses.
The supplementation started on the day of oocyte retrieval, and continued for 14 days after ET (On the day of the β-hCG test). If the pregnancy test was negative we discontinued the treatment but if the pregnancy was achieved we continued the support until 8 weeks gestation. We followed up all the pregnant women for at least 12 weeks gestation.
Main outcome measures: The main outcome measures were: implantation rate (number of gestational sacs to the number of embryos transferred), chemical pregnancy, clinical pregnancy (visualization of intra-uterine gestational sac and fetal heart beat by U/S by 6th weeks), ongoing pregnancy (Pregnancy that continued beyond 12 weeks of gestation) or early pregnancy loss (pregnancy loss occurring before 12 weeks of gestation)), and multiple gestation.
Statistical analysis: Data of the 180 participant women were entered into computerized software with The Statistical Package for the Social Sciences (SPSS) was utilized for statistical analysis. Chi-square tests were applied to compare the frequencies of categorical variables. Such as age groups, BMI categories, type and causes of infertility, pregnancy rates, type of gestation and abortion.
Student t test, independent two samples model, was used to compare mean values between the studied groups including mean age, mean BMI, duration of infertility, hormonal levels, embryological parameters, Fertilization rates, Cleavage rates and Implantation rates. Level of significance of ≤ 0.05 considered significant difference.
Results
A total of 180 women underwent ICSI/ET participated in this comparative study. We randomly divided them into two studied groups according to the modes of luteal support. Progesterone/estrogen group (P/E2; No. 90) and progesterone only group (P; No. 90). No statistically significant differences had been found between both groups in regards to the age, body mass index BMI, in addition to the type, causes and duration of subfertility (P> 0.05) as shown in Tab. 1.
| Variables | P/E2 | P | P value | |
|---|---|---|---|---|
| Age (years) | Mean ± SD* | 29.4 ± 4.2 | 29,0 ± 5.4 | 0.59 |
| BMI Kg/m2 | Mean ± SD* | 26.1 ± 3.1 | 25.8 ± 3.5 | 0.48 |
| Duration of subfertility | Mean ± SD* | 7.2 ± 2.1 | 7.7 ± 4.0 | 0.30 |
| Type of subfertility % | Primary | 67 (74.4%) | 69 (76.7%) | 0.73 |
| Secondary | 23 (25.6%) | 21 (23.3%) | ||
| Cause of subfertility % | Female factor | 41 (45.6%) | 43 (47.8%) | 0.90 |
| Male factor | 34 (37.7%) | 31 (34.4%) | ||
| Mixed | 5 (5.6%) | 7 (7.8%) | ||
| Unexplained | 10 (11.1%) | 9 (10.0%) | ||
Tab. 1. Baseline characteristics of the studied groups.
Similarly, no significant differences were observed in hormonal assays on cycle day 2, including FSH, LH, and E2. Additionally, there were no significant differences in E2 levels on the day of hCG administration on E2 and progesterone hormones in both studied groups P>0.05 Tab. 2.
| Variables | P / E2 | P | P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| CD2 FSH | 4.88 ± 2.03 | 5.28 ± 2.00 | 0.19 |
| CD2 LH | 3.10 ± 2.08 | 3.47 ± 2.20 | 0.26 |
| CD2 E2 | 38.32 ± 16.48 | 40.26 ± 16.18 | 0.43 |
| Day hCG E2 | 2130.70 ± 798.06 | 2101.86 ± 829.92 | 0.82 |
| Day hCG progesterone | 1.28 ± 0.44 | 1.19 ± 0.39 | 0.27 |
Tab. 2. Mean hormonal levels of the studied groups.
Tab. 3. compares the mean values of different embryological parameters between the two groups with respect to the number of follicles (P=0.34), number of MII oocytes (P=-.51), The number of 2PN oocytes (P=0.15), fertilization rate (P=0.56), total number of embryos (P=0.19), cleavage rate (P=0.72), number of good-quality embryos (i.e., grade 1 embryos) (P=0.30), and the number of embryos transferred (P=0.34) showed no significant differences.
| Variables | P / E2 | P | P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Number of follicle | 11.3 ± 4.6 | 10.7 ± 4.0 | 0.34 |
| Number of MII | 8.8 ± 4.5 | 8.4 ± 4.2 | 0.51 |
| Number of 2PN | 6.1 ± 3.2 | 5.4 ± 2.8 | 0.15 |
| Fertilization rate* | 69.4% | 64.1% | 0.56 |
| Number of embryos | 5.1 ± 2.3 | 4.6 ± 2.6 | 0.19 |
| Cleavage rate* | 57.9% | 54.2% | 0.72 |
| Number of grade 1 embryo | 4.3 ± 2.4 | 3.9 ± 2.3 | 0.30 |
| Number of embryo transfer | 2.9 ± 0.7 | 2.7 ± 0.8 | 0.34 |
Tab. 3. Embryological parameters of the studied groups.
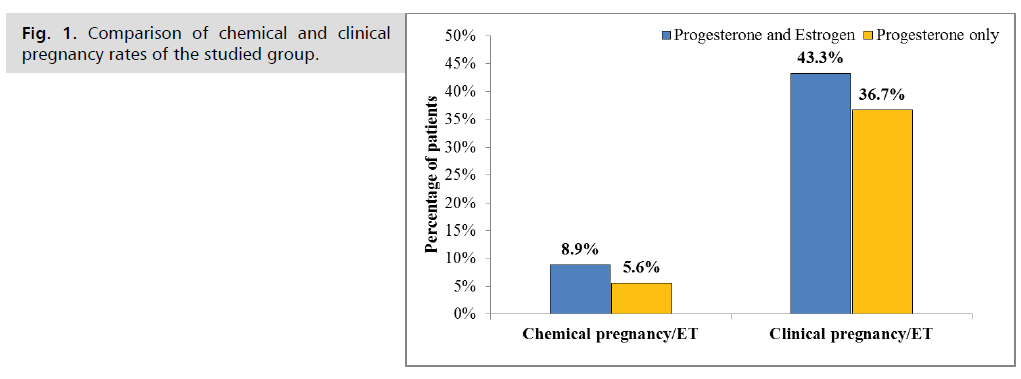
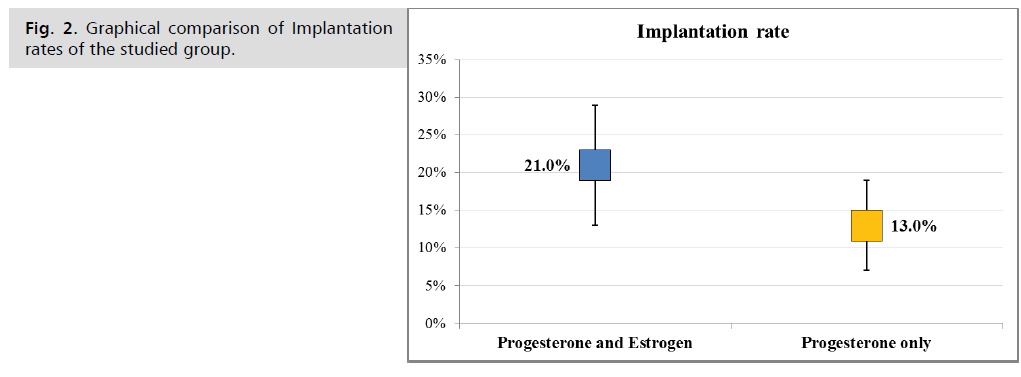
Pregnancy outcomes between the two studied groups were compared and summarized in Tab. 4. The chemical and clinical pregnancy rates in the estrogen group were slightly higher compared to the progesterone-only group, but without a statistical significant (8.9% vs. 5.6% and 43.3% vs. 36.7% respectively) Fig. 1., similarly, a slightly higher but not significant in ongoing pregnancy rate was noted in estrogen group as compared with progesterone only group (38.9% vs. 33.3%, P= 0.82). The multiple pregnancy rate was higher in the estrogen group at 33.3%, compared to that of progesterone only group 15.2%, but still without a statistical significant (P= 0.25). Nevertheless, the mean value and the range of the implantation rate was higher in estrogen/progesterone group as compared with that of the progesterone only group which was statistically significant (21% vs. 13%, p<0.001), Fig. 2.
| Variables | P / E2 | P | P value | |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Chemical pregnancy/ET | 8 (8.9%) | 5 (5.6%) | 0.56 | |
| Clinical pregnancy/ET | 39 (43.3%) | 33 (36.7%) | 0.44 | |
| Ongoing pregnancy/ET | 35 (38.9%) | 30 (33.3%) | 0.82 | |
| Early pregnancy loss* | 4/39 (10.3%) | 3/33 (9.1%) | ||
| Implantation rate | 21.0% | 13.0% | <0.001 | |
| Gestation type* | Single | 26 (66.7%) | 28 (84.8%) | 0.25 |
| Multiple | 13 (33.3%) | 5 (15.2%) | ||
Tab. 4. Pregnancy outcome of the studied groups.
Fig. 1. Comparison of chemical and clinical pregnancy rates of the studied group.
Fig. 2. Graphical comparison of Implantation rates of the studied group.
Discussion
Luteal phase support (LPS) is a critical component following IVF and ICSI cycles due to the luteal phase deficiency commonly associated with these procedures. In IVF and ICSI, supra-physiologic levels of sex steroids, resulting from ovarian hyperstimulation, suppress LH secretion, leading to a reduction in the production of estrogen and progesterone during the luteal phase. To date, progesterone administration remains the standard approach for luteal phase support [17,18]. The role of progesterone for luteal support in stimulated IVF cycles is well-established. However, the potential benefits of additional supplementation with estradiol (E2) and other medications remain a topic of ongoing debate [19]. This study aims to evaluate the effect of adding oral estradiol (4 mg) to progesterone for luteal phase support, in comparison to progesterone alone, in patients undergoing ICSI cycles.
There were no significant differences of selected parameters between the two studied groups include: the age, body mass index in addition to type, cause and duration of subfertility. (in all comparison p value>0.05).
there were no statistically significant differences in the hormonal levels, in all comparisons, (P>0.05). as well as Insignificant difference between both groups in respect to number of follicles, number of mature oocytes, total number and grade 1 embryos, and the number of embryos transferred. Similarly, fertilization and cleavage rates were not significantly difference in both groups (P value<0.05). These finding supported by Fatemi et al. [20], Marzeih A. et al. [21], and Mohammed EL-Mahdy et al. [22].
In the present study oral estradiol added to progesterone in a dose of 4 mg at day of oocytes retrieval and the pregnancy outcomes were evaluated, including chemical, clinical, and ongoing pregnancy rates, as well as implantation rate, multiple gestations, and early pregnancy loss.
The implantation rate/embryo transfer it was 21% for E2 plus progesterone group which was statically significantly higher than the 13% for progesterone alone group (P value<0.001). This finding refuted by Serna et al. [23]. They evaluated the effect of transdermal E2 after embryo transfer on cycle outcomes and found no difference between these luteal phase support (LPS) protocols in terms of implantation rate, ongoing pregnancy rate, early pregnancy loss, or multiple pregnancy rates.
Fatemi et al. [20] they determined that there were no statistically significant differences in implantation and pregnancy rates when using oral E2 with progesterone as LPS.
We found that the chemical pregnancy, clinical pregnancy, and ongoing pregnancy rates were relatively higher in E2 and progesterone group than those with progesterone only group. However, it did not achieve statistical significance (P<0.05). This agrees with Ceyan et al. [24] which conclude that pregnancy rates and ongoing pregnancy rates were similar with and without estrogen supplementation.
The number of multiple gestations is higher in estradiol and progesterone group than progesterone only group but not reaches the statically significant (P<0.05). Lastly, clinical early miscarriage was not significantly different between both groups.
All the above findings supported by Ashraf M et al. [25] and Ismael Madkour et al. [26]. Both studies indicated that luteal phase supplementation with oral E2 and progesterone, when combined with the GnRH-antagonist protocol, did not result in any significant improvements. There were no notable differences in pregnancy rates, ongoing pregnancy rates, implantation rates, or abortion rates. Another meta-analysis published by Huang et al. [16]. They demonstrated that adding E2 during Luteal Phase Support (LPS) does not enhance ICSI outcomes, regardless of the daily dosage or the method of administration. Xiao et al meta-analysis in 2015 27 identified 11 articles using estrogen supplementation in oral, vaginal, and trans-dermal forms They demonstrated a significant advantage of combining E2 with progesterone compared to using progesterone alone regarding clinical pregnancy only, but no significant was found between the two groups for the ongoing pregnancy rate, fertilization rate, implantation rate, and miscarriage rate [27].
Various outcomes were reported in a systematic review done by Lanna MA et al. [28] revealed that Only one study indicates successful implantation rate in patients using E2 with progesterone for LPS in ICSI cycles used GnRH-antagonist protocol as ovarian stimulation. However, the success was not confirmed in any of the selected studies regarding pregnancy rates.
Olso Zhao and colleagues, in a retrospective cohort study, concluded that the impact of adding E2 for luteal phase support was dependent on the E2 levels on the hCG trigger day. E2 supplementation was associated with improved outcomes in patients with low E2 levels, but it proved detrimental in those with high E2 levels on the trigger day. They found that E2 supplementation significantly increased the live birth rate in cases where E2 levels were below 5,000 pmol/L on the day of hCG trigger [4]. As well as Kutlusoy and colleagues assessed the combination of E2 and progestin in IVF cycles involving poor responder patients. They demonstrated that adding 2 mg/day of E2 to progesterone for luteal phase support significantly increased the clinical pregnancy rate in these patients [29].
It seems that the optimal E2 level in luteal phase is not the same in different patients and subgroup of patients could potentially benefit from this approach, rather than administering E2 universally.
Conclusion
Addition of oral E2 in a dose of 4mg to progesterone in patients undergo ICSI did not significantly improve pregnancy outcomes. However, the implantation rate significantly increased.
Recommendations
A larger multicenter cohort study is recommended to further clarify the role of luteal E2 supplementation in IVF and to investigate the optimal regimen, including the appropriate dose and administration route.
Authors' Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Gelbaya TA, Kyrgiou M, Tsoumpou I, et al. The use of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection cycles: a systematic review and meta-analysis. Fertil Steril. 2008;90(6):2116-2125.
- Olooto WE, Amballi AA, Banjo TA. A review of Female Infertility; important etiological factors and management. J Microbiol Biotech Res. 2012;2(3):379-85.
- Dashti S, Eftekhar M. Luteal-phase support in assisted reproductive technology: An ongoing challenge. Int J Reprod BioMed. 2021;19(9):761.
- Zhao J, Hao J, Li Y. Individualized luteal phase support after fresh embryo transfer: unanswered questions, a review. Reprod Health. 2022;19(1):19.
- Wu H, Zhang S, Lin X, et al. Luteal phase support for in vitro fertilization/intracytoplasmic sperm injection fresh cycles: a systematic review and network meta-analysis. Reprod Biol Endocrinol. 2021;19:1-1.
- Shoham G, Leong M, Weissman A. A 10-year follow‐up on the practice of luteal phase support using worldwide web‐based surveys. Reprod Biol Endocrinol. 2021;19:1-1.
- Simon V, Robin G, Keller L, et al. Systematic use of long-acting intramuscular progesterone in addition to oral dydrogesterone as luteal phase support for single fresh blastocyst transfer: A pilot study. Front Endocrinol. 2022;13:1039579.
- Tomic V, Tomic J, Klaic DZ, et al. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015;186:49-53.
- Umesh M Jindal, Swati Verm. Luteal phase support: Kanini Arao, Howard Carp, Robert Fischer. Principle and Practice of Assisted Reproductive Technology Volume 1 Infertility. 1st edition India;2014: 622-628.
- Humaidan P, Thomsen LH, Alsbjerg B. GnRHa trigger and modified luteal support with one bolus of hCG should be used with caution in extreme responder patients. Hum Reprod. 2013;28(9):2593-2594.
- Vanetik S, Segal L, Breizman T, et al. Day two post retrieval 1500 IUI hCG bolus, progesterone-free luteal support post GnRH agonist trigger–a proof of concept study. Gynecol Endocrinol. 2018;34(2):132-135.
- van der Linden M, Buckingham K, Farquhar C, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2011(10).
- Pirard C, Donnez J, Loumaye E. GnRH agonist as luteal phase support in assisted reproduction technique cycles: results of a pilot study. Hum Reprod. 2006;21(7):1894-1900.
- Maghraby H, Abdelbadie AS, Aboali A, et al. GnRH agonist as a luteal support in IVF cycle: mini-review—is there a role?. Middle East Fertil Soc J. 2022;27(1):18.
- Drakakis P, Loutradis D, Vomvolaki E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection–embryo transfer. Gynecol Endocrinol. 2007;23(11):645-652.
- Huang N, Situ B, Chen X, et al. Meta-analysis of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2015;103(2):367-373.
- Tavaniotou A, Albano C, Smitz J, et al. Comparison of LH concentrations in the early and mid-luteal phase in IVF cycles after treatment with HMG alone or in association with the GnRH antagonist Cetrorelix. Hum Reprod. 2001;16(4):663-667.
- Aboulghar M. Luteal support in reproduction: when, what and how?. Curr Opin Obstet Gynecol. 2009;21(3):279-284.
- ESHRE Guideline Group on Ovarian Stimulation, Bosch E, Broer S, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020;2020(2):hoaa009.
- Fatemi HM, Kolibianakis EM, Camus M, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Hum Reprod. 2006;21(10):2628-2632.
- Aghahosseini M, Aleyassin A, Khodaverdi S, et al. Estradiol supplementation during the luteal phase in poor responder patients undergoing in vitro fertilization: a randomized clinical trial. J Assist Reprod Genet. 2011;28:785-790.
- Abdel-Moneim ME, El-Agwany AS, Ali AH. Is There a Role for Estradiol With Progesterone in Luteal Phase Support With Intracytoplasmic Sperm Injection Cycles? A Retrospective Controlled Study With Reviewing the Literature. J Clin Obstet Gynaecol. 2015;4(2):226-231.
- Serna J, Cholquevilque JL, Cela V, et al. Estradiol supplementation during the luteal phase of IVF-ICSI patients: a randomized, controlled trial. Fertil Steril. 2008;90(6):2190-2195.
- Ceyhan ST, Basaran M, Duru NK, et al. Use of luteal estrogen supplementation in normal responder patients treated with fixed multidose GnRH antagonist: a prospective randomized controlled study. Fertil Steril. 2008;89(6):1827-1830.
- Moini A, Modarress SZ, Amirchaghmaghi E, et al. The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles–a randomized controlled study. Arch Med Sci. 2011;7(1):112-116.
- Ismail Madkour WA, Noah B, Abdel Hamid AM, et al. Luteal phase support with estradiol and progesterone vs. progesterone alone in GnRH antagonist ICSI cycles: a randomized controlled study. Hum Fertil. 2016;19(2):142-149.
- Zhang XM, Lv F, Wang P, et al. Estrogen supplementation to progesterone as luteal phase support in patients undergoing in vitro fertilization: systematic review and meta-analysis. Medicine. 2015;94(8):e459.
- Pinheiro LM, da Silva Cândido P, Moreto TC, et al. Estradiol use in the luteal phase and its effects on pregnancy rates in IVF cycles with GnRH antagonist: a systematic review. JBRA Assist Reprod. 2017;21(3):247.
- Kutlusoy F, Guler I, Erdem M, et al. Luteal phase support with estrogen in addition to progesterone increases pregnancy rates in in vitro fertilization cycles with poor response to gonadotropins. Gynecol Endocrinol. 2014;30(5):363-366.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Amal Muner Mubarak*, Aseel Abdulameer Mohammed, Dalal M Jarrah and Zahraa F. AlhilliCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.