Research - (2025) Volume 20, Issue 1
The effect of DHEA on ovarian reserve markers in patients with diminished ovarian reserve
Rabab Awad Farhood, Vian Hussam Almansi Alqani* and Ashwaq Kadhim MohammedReceived: 03-Feb-2025, Manuscript No. gpmp-25-160889; Editor assigned: 05-Feb-2025, Pre QC No. P-160889; Reviewed: 17-Feb-2025, QC No. Q-160889; Revised: 24-Feb-2025, Manuscript No. R-160889; Published: 31-Mar-2025
Abstract
Background: The purpose of this study was to look at how DHEA affects ovarian reserve parameters in patients with low ovarian reserve. At the end of the experiment, a significant number of women were able to become pregnant. We also noticed differences in blood hormone levels between pregnant and non-pregnant women.
Aim of the study: The purpose of this study was to see how DHEA affected ovarian reserve indices in those who had low reserves.
Patients and methods: In the department of obesteric and gynecology at Adiwaniyah Maternity and Pediatirc teaching Hospital, From februrary 2023 to october 2023, in this study 30 infertile women with poor ovarian reserve were enrolled they were treated using DHEA and followed up for 6 months by evaluating pregnancy outcome and changes in serum hormonal levels including FSH, LH, estradiol, testosterone, AMH and DHEA.
Results: In this study, five patients (16.7%) succeeded in achieving pregnancy. The mean age of pregnant women was lower than that of non-pregnant women, 27.20 ± 1.92 years vs. 31.12 ± 4.64 years, respectively, but the difference in mean age did not reach statistical significance (p=0.057). There was no significant difference in mean body mass index between pregnant and non-pregnant women, 23.90 ± 1.28 kg/m2 vs. 25.01 ± 1.91 kg/m2, respectively (p=0.090). There was also no significant difference in duration of infertility between pregnant and non-pregnant women, 3.00 ± 0.71 years vs. 3.72 ± 1.28 years, respectively (p=0.251).
Conclusions: The results of this study revealed that treatment with DHEA can improve ovarian reserve markers and pregnancy outcome in women with poor ovarian reserve.
Keywords
DHEA; Ovarian reserve; Markers; Non-pregnant women
Introduction
Diminished Ovarian Reserve (DOR) encounter a major hurdle, in the field of reproductive medicine. This condition is marked by a decrease in both the number and quality of eggs. Individuals, with DOR frequently encounter fertility results despite resorting to assisted techniques like In Vitro Fertilization (IVF) [1-4]. The Development of Ovarian Reserve (DOR) is influenced by factors, including genetic predisposition, older maternal age previous ovarian surgeries, chemotherapy treatment and unknown reasons [5]. In settings DOR is commonly identified using biomarkers, like Follicle Count (AFC) anti Müllerian Hormone (AMH) levels and basal Follicle Stimulating Hormone (FSH) levels [6,7]. These indicators offer information, about the ovarian reserve aiding in the prediction of how the ovaries will respond to stimulation and the overall reproductive capacity [8,9].
There are clinical aspects to the concept of ovarian reserve. Biologically, ovarian reserve relates to the number of eggs in the ovaries, which drops with age. In the middle 30s, this slows down. Shows more clearly by the late 30s. Ovarian reserve is assessed in terms of the likelihood of pregnancy and the response of the ovaries to ART treatment. Though medicine has advanced, there is currently no way to stop or reverse the ovarian reserve drop [10-12].
Dehydroepiandrosterone (DHEA) is a steroid hormone that acts as a building block, for female hormones. Recent research suggests that taking DHEA supplements could positively impact markers of health and fertility outcomes in women, with Ovarian Reserve (DOR). The proposed mechanisms involve boosting the recruitment of follicles enhancing the function of granulosa cells and improving the quality of eggs. It is believed that DHEA achieves these effects by leveraging its properties potentially adjusting the environment within the ovaries to support follicle development [13].
The effectiveness of DHEA supplementation is still uncertain despite the increasing interest, in it. Some research shows outcomes like ovarian reserve markers and higher pregnancy rates but other studies do not show clear advantages. The differences in how the studiesre conducted the dosages used and the types of patients involved all play a role in these results. Consequently more studies are required to understand how DHEA can be helpful, in treating ovarian reserve [14].
This research intends to explore how DHEA impacts the markers of ovarian reserve in individuals, with Ovarian Reserve (DOR). By observing variations in AFC, AMH and basal FSH levels pre and post DHEA intake we aim to gain a grasp of its healing advantages. Furthermore we will assess real life results like egg production, fertilization rates and chances of pregnancy to understand the effects of using DHEA, for this group of patients.
Patients and Methods
Study design and sample size
The current prospective cohort study was conducted in obstetrics, gynecology department and emergency department in Al-Diawania Maternity and Pediatric Teaching Hospital, Iraq. The study period extended from Februrary 2023 to october 2023. The study enrolled 30 infertile women aged 25-40 years old.
Ethical consideration
This study was approved by Iraqi Board for Medical Specilization and Ethical Approval Committee of the Teaching Hospital. Written consent was obtained from all patients participating in the current study before being enrolled in the protocol.
Inclusion criteria
• Age between 25 to 40 years
• Infertile women
Exclusion criteria
• Women with chronic medical illness
• Women with contra-indications to DHEA
• Women who refused to be enrolled in this study
Methods
In this study 30 infertile women with poor ovarian reserve were given combined oral DHEA. Serum levels of FSH, LH, AMH, estradiol, testosterone and DHEA were assessed at baseline, three months and after 6 months. Body mass index was also calculated at baseline.
Statistical analysis
The data was collected, translated to a spreadsheet in Microsoft Office Excel 2010, and then loaded into SPSS (Statistical Package for the Social Sciences) version 23. The numeric quantitative data were provided as mean, range, and Standard Deviation (SD), whereas the qualitative data were presented as number and percentage. An independent sample t-test was used to compare the means of any two groups, whilst a chi-square test was used to examine the relationship between any two categorical variables. The criterion of significance was fixed at P<0.05.
Results
Table 1 shows the overall characteristics of the women who participated in this study. Five of the trial's participants, or 16.7%, were able to conceive a child. Pregnant women had a lower average age (27.20 ± 1.92 years) than non-pregnant women (31.12 ± 4.64 years). However, the difference in average age was not statistically significant (p=0.057).
There was no significant difference in average body mass index (BMI) between pregnant and non-pregnant women. Pregnant women's mean BMI was 23.90 ± 1.28 kg/m2, whereas non-pregnant women's was 25.01 ± 1.91 kg/m2 (p=0.090). There was no significant difference in the duration of infertility between pregnant and non-pregnant women. The average duration of infertility for pregnant women was 3.00 ± 0.71 years, while for non-pregnant women it was 3.72 ± 1.28 years (p=0.251) (Tab. 1.).
| Characteristic | Total n=30 |
Positive Pregnancy n=5 |
Negative Pregnancy n=25 |
p |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 30.47 ± 4.53 | 27.20 ± 1.92 | 31.12 ± 4.64 | 0.057 M NS |
| Range | 25-40 | 25-30 | 25-40 | |
| BMI (kg/m2) | ||||
| Mean ± SD | 24.82 ± 1.85 | 23.90 ± 1.28 | 25.01 ± 1.91 | 0.290 M NS |
| Range | 22-28.9 | 22-25.1 | 22.2-28.9 | |
| Duration of infertility (years) | ||||
| Mean ± SD | 3.60 ± 1.22 | 3.00 ± 0.71 | 3.72 ± 1.28 | 0.251 M NS |
| Range | 2-6 | 2-4 | 2-6 | |
Tab. 1. The general characteristics of women enrolled in this study.
Table 2 shows a comparison of serum hormonal levels in pregnant and non-pregnant women. Pregnant women had significantly lower serum FSH levels than non-pregnant women before, three, and six months after therapy (p<0.001).
Pregnant women had substantially lower serum LH levels than non-pregnant women before, three, and six months after therapy (p<0.001).
Pregnant women had substantially lower serum AMH levels than non-pregnant women before, three, and six months after therapy (p<0.001).
Pregnant women had significantly lower serum estradiol levels than non-pregnant women before, three, and six months after therapy (p<0.001).
Pregnant women had substantially lower serum DHEA levels than non-pregnant women before, three, and six months after therapy (p<0.001).
Pregnant women had substantially lower serum testosterone levels than non-pregnant women before, three, and six months after therapy (p<0.001).
Changes in these hormonal levels after treatment in comparison with pre-treatment level are shown in Figures 1 through 6 (Tab. 2.) and (Fig. 1.-Fig. 6.).
| Characteristic | Time | Total n=30 |
Positive Pregnancy n=5 |
Negative Pregnancy n=25 |
P |
|---|---|---|---|---|---|
| FSH (mIU/ml) | Before treatment | 36.10 ± 14.63 | 18.52 ± 2.70 | 39.62 ± 13.42 | <0.001 M *** |
| 3 months later | 14.21 ± 5.26 | 8.48 ± 0.66 | 15.35 ± 5.02 | <0.001 M *** | |
| 6 months later | 14.30 ± 4.39 | 9.12 ± 0.75 | 15.33 ± 4.06 | <0.001 M *** | |
| LH (mIU/ml) | Before treatment | 13.99 ± 5.84 | 7.05 ± 1.04 | 15.37 ± 5.38 | <0.001 M *** |
| 3 months later | 7.74 ± 2.53 | 5.05 ± 0.29 | 8.28 ± 2.43 | <0.001 M *** | |
| 6 months later | 8.74 ± 3.39 | 5.17 ± 0.37 | 9.46 ± 3.27 | <0.001 M *** | |
| AMH (ng/ml) | Before treatment | 1.13 ± 0.36 | 0.69 ± 0.07 | 1.22 ± 0.33 | <0.001 M *** |
| 3 months later | 1.53 ± 0.55 | 0.83 ± 0.13 | 1.67 ± 0.49 | <0.001 M *** | |
| 6 months later | 1.64 ± 0.63 | 0.85 ± 0.14 | 1.79 ± 0.56 | <0.001 M *** | |
| Estradiol (pg/ml) | Before treatment | 37.52 ± 7.88 | 27.74 ± 1.64 | 39.48 ± 7.12 | <0.001 M *** |
| 3 months later | 47.69 ± 21.62 | 21.05 ± 4.37 | 53.02 ± 19.60 | <0.001 M *** | |
| 6 months later | 40.85 ± 14.21 | 22.97 ± 3.10 | 44.42 ± 12.74 | <0.001 M *** | |
| DHEA (ng/ml) | Before treatment | 1.35 ± 0.49 | 0.75 ± 0.09 | 1.47 ± 0.45 | <0.001 M *** |
| 3 months later | 5.65 ± 2.00 | 3.26 ± 0.36 | 6.13 ± 1.84 | <0.001 M *** | |
| 6 months later | 7.07 ± 2.20 | 4.34 ± 0.46 | 7.62 ± 1.99 | <0.001 M *** | |
| Testosterone (nmol/L) | Before treatment | 0.78 ± 0.22 | 0.50 ± 0.05 | 0.84 ± 0.20 | <0.001 M *** |
| 3 months later | 0.85 ± 0.28 | 0.50 ± 0.06 | 0.92 ± 0.25 | <0.001 M *** | |
| 6 months later | 0.88 ± 0.28 | 0.53 ± 0.06 | 0.96 ± 0.26 | <0.001 M *** |
Tab. 2. Comparison of serum hormonal levels between women who achieved pregnancy and non-pregnant women.
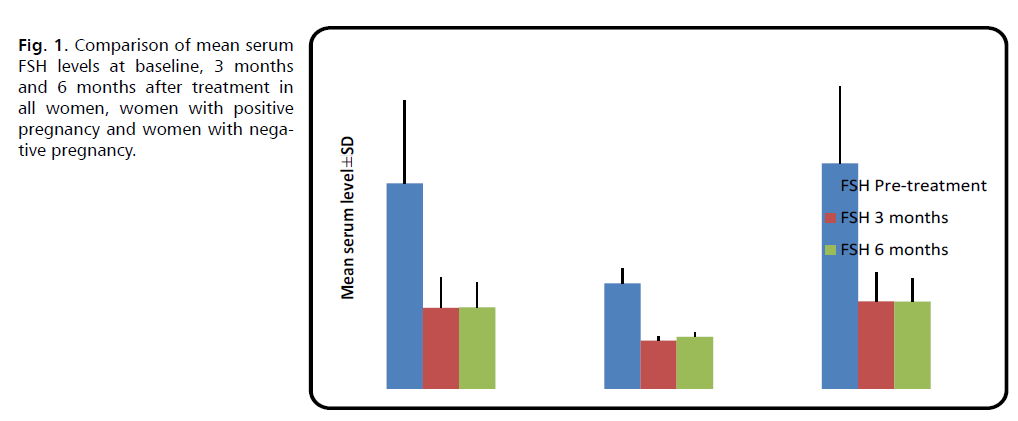
Fig. 1. Comparison of mean serum FSH levels at baseline, 3 months and 6 months after treatment in all women, women with positive pregnancy and women with negative pregnancy.
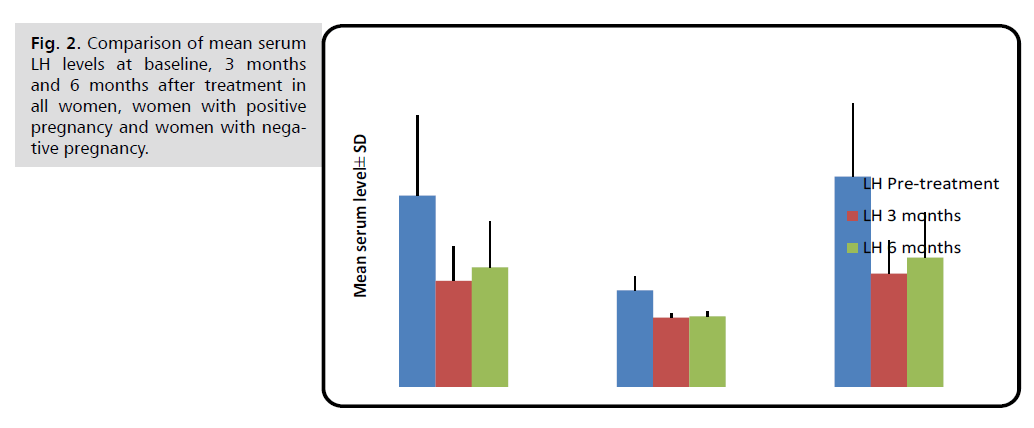
Fig. 2. Comparison of mean serum LH levels at baseline, 3 months and 6 months after treatment in all women, women with positive pregnancy and women with negative pregnancy.
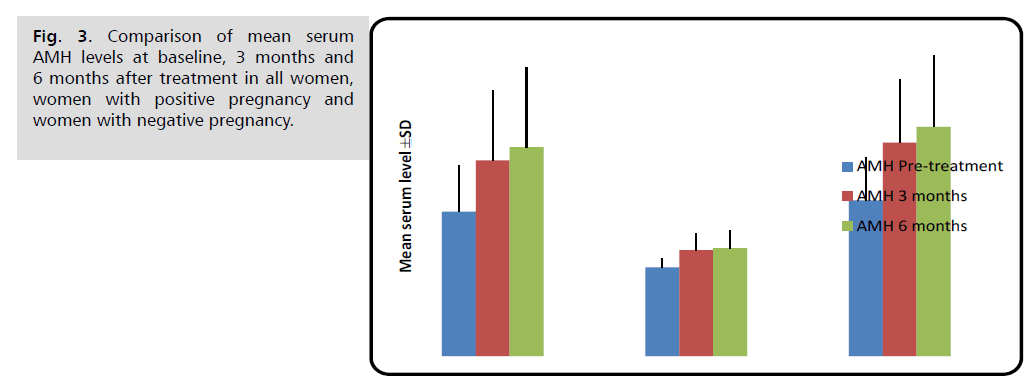
Fig. 3. Comparison of mean serum AMH levels at baseline, 3 months and 6 months after treatment in all women, women with positive pregnancy and women with negative pregnancy.
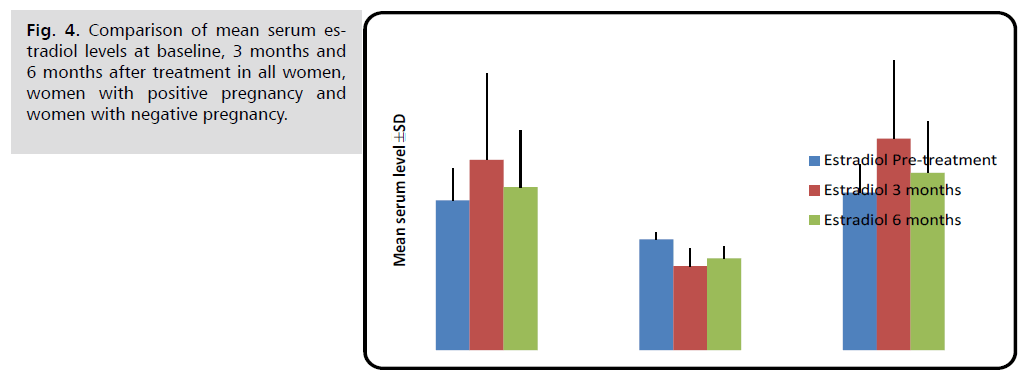
Fig. 4. Comparison of mean serum estradiol levels at baseline, 3 months and 6 months after treatment in all women, women with positive pregnancy and women with negative pregnancy.
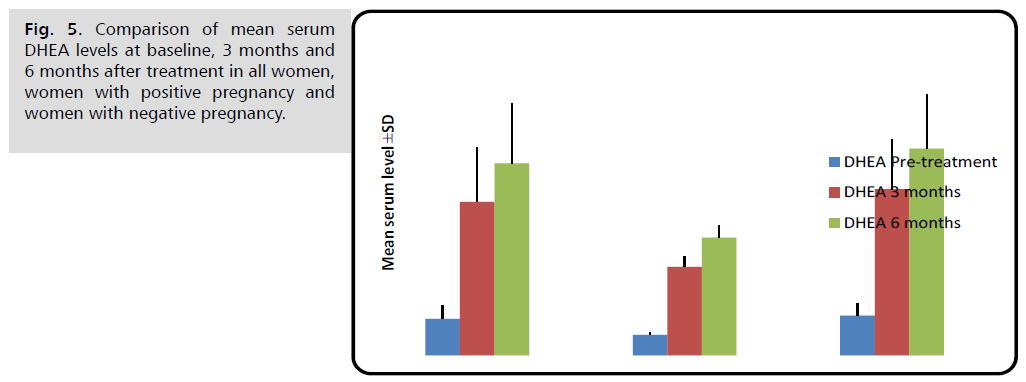
Fig. 5. Comparison of mean serum DHEA levels at baseline, 3 months and 6 months after treatment in all women, women with positive pregnancy and women with negative pregnancy.
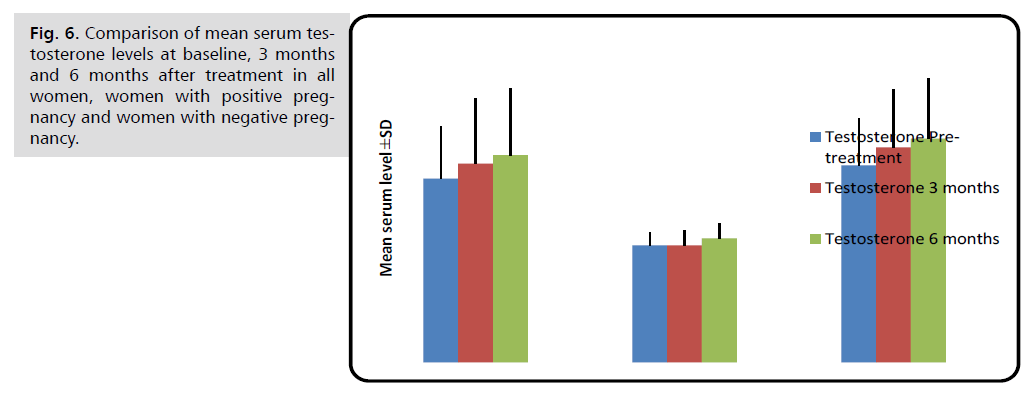
Fig. 6. Comparison of mean serum testosterone levels at baseline, 3 months and 6 months after treatment in all women, women with positive pregnancy and women with negative pregnancy.
Discussion
This study examined the impact of DHEA on indicators of ovarian reserve in patients with decreased ovarian reserve. At the conclusion of the trial, a large number of women were able to successfully achieve pregnancy. Furthermore, we noted alterations in blood hormone levels between women who were pregnant and those who were not pregnant.
Schuh-Huerta, et al. [15] preformed a study to investigate whether DHEA supplementation influenced ovarian reserve metrics and pregnancy rates in individuals with Poor Ovarian Response (POR) and Primary Ovarian Insufficiency (POI). They concluded that oral DHEA supplementation boosts ovarian reserve and pregnancy rates in women with POR. These findings of improved pregnancy rates and ovarian reserve metrics are consistent with our observations.
Androgen insufficiency has been proposed as the primary underlying issue in some infertile individuals with Diminished Ovarian Reserve (DOR) [2-4]. Administering androgen supplements, such as testosterone or DHEA, may enhance the initial growth of follicles and enhance the functional capacity of the ovaries in these individuals [5-7]. In order to harvest more fully developed oocytes during oocyte collection, ovarian stimulation is concentrated on the final two weeks, which are marked by the existence of antral follicles.
Unlike conventional ovarian stimulation protocols, stimulation and synchronization of follicles in the earlier period of the maturation process will improve outcomes in patients with POR [8]. Recent animal studies have shown that androgens induce follicles in the preantral and antral stages through granulosa cells [5,6]. Furthermore, in the early stages of follicular maturation, androgens and FSH have been shown to exhibit synergistic effects on granulosa cells. In the present study, we clearly demonstrated that DHEA supplementation markedly improved the ovarian-reserve markers in patients with POR.
The findings of our research are inconsistent with the results of prior investigations. In their study, Steiner, et al. [16] found that supplementing with DHEA at a dosage of 80 mg per day for a period of 2 to 5 months resulted in an enhanced ovarian response to gonadotropins in patients with poor ovarian response (POR). This improvement was attributed to an increase in the activity of insulin-like growth factor 1 (IGF-1). Similarly, Tal, et al. [10] found that administering DHEA at a dosage of 75 mg per day over a period of 1-4 months in women with Poor Ovarian Response (POR) leads to an elevation in serum anti-Müllerian hormone (AMH) levels. Abdalla, et al. [17] demonstrated that blood levels of anti-Müllerian hormone (AMH), inhibin B, and antral follicle count (AFC) were elevated following the administration of 25 mg/day dehydroepiandrosterone (DHEA) for a minimum of 6 weeks, whereas Follicle-Stimulating Hormone (FSH) and Estradiol (E2) levels fell.
Broer, et al. [18] found that DHEA had no favorable impact on ovarian reserve indicators in women with POR. Differences in these trials might be explained by clinical heterogeneity due to the small number of cases, variance in age, difference in patient demographic characteristics, and variation in treatment duration. It has been shown that DHEA supplementation improves ovarian reserve indicators in patients with poor ovarian response.
DHEA application intervals range from 6 weeks to a year. In this investigation, we discovered substantial differences in AMH levels before and after therapy. In contrast to our findings, several studies indicate that DHEA has no influence on AMH levels. Only a few isolated studies have shown an increase in AMH levels following DHEA [11].
Similarly, Jayaprakasan, et al. [19] linked the beneficial impact of DHEA supplementation on ovarian reserve to significantly changed gene expression in the ovaries. Elgindy, et al. [20] demonstrated for the first time that DHEA supplementation enhances endometrial HOXA-10 mRNA expression in poor responders. DHEA supplementation has been shown to minimize the risk of spontaneous abortion when compared to those who did not take it. Jung, et al. [21] found a 15.1% mean risk of miscarriage following DHEA therapy.
Previous research has demonstrated that DHEA supplementation boosts clinical and cumulative pregnancy rates in individuals with poor ovarian response. The clinical pregnancy rate in poor responders getting DHEA supplementation was 28.1% in a trial by Liu, et al. [22], and 23.5% in a study by Penarrubia, et al. [23].
The clinical pregnancy rate in individuals with poor ovarian response receiving DHEA supplementation was reported to be 32% in a research by Jung, et al. [21], 2.1% by Tal, et al. [24], and 18.8% by Yeung, et al. The clinical pregnancy rate in our patient population was 16.7%.
The ability of women to conceive is known to decrease with aging. This decrease starts around the age of 32 years and accelerates after 37 years [23,24]. No previous study could be found which had evaluating pregnancy outcomes by age in patients with POR who received DHEA supplementation. Nonetheless, Gleicher, et al. showed that DHEA supplementation improved the ovarian reserve markers, especially in younger patients and this is supportive to our findings. Better pregnancy outcomes might be expected in younger patients with better improvement of ovarian reserves.
Conclusion
Treatment with DHEA can improve ovarian reserve markers and pregnancy outcome in infertile women with poor ovarian reserve.
Significant changes in serum hormonal levels can be induced by the use of DHEA in women with poor ovarian reserve, but these changes are in favor of these women and have negligible side effects.
Recommendations
Larger sample size and multicenter study is needed to validate the results of the current study.
DHEA treatment can be used in clinical practice to improve poor ovarian reserve in women undergoing controlled ovarian stimulation for primary infertility.
References
- Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder—a plea for universal definitions. J Assist Reprod Genet. 2015;32:1709-1712.
- De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. The Lancet. 2010;376(9744):911-921.
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11(4):391-410.
- Ferraretti A, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of ‘poor response'to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Wang J, Pan X, Zhou J, et al. Advances in understanding the effect and mechanism of dehydroepiandrosterone on diminished ovarian reserve. Drug Discov Ther. 2023;17(2):87-94.
- Ozelci R, Aldemir O, Dilbaz S, et al. The impact of different etiologies of diminished ovarian reserve on pregnancy outcome in IVF-ET cycles. Turk J Med Sci. 2019;49(4):1138-1144.
- Kaur M, Arora M. Diminished ovarian reserve, causes, assessment and management. Int J Infertil Fetal Med. 2013;4(2):45-55.
- Ulrich ND, Marsh EE. Ovarian reserve testing: a review of the options, their applications, and their limitations. Clin Obstet Gynecol. 2019;62(2):228-237.
- Peck JD, Quaas AM, Craig LB, et al. Lifestyle factors associated with histologically derived human ovarian non-growing follicle count in reproductive age women. Hum Reprod. 2016;31(1):150-157.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Hansen KR, Knowlton NS, Thyer AC, et al. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23(3):699-708.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-17.
- Daney de Marcillac F, Pinton A, Guillaume A, et al. What are the likely IVF/ICSI outcomes if there is a discrepancy between serum AMH and FSH levels? A multicenter retrospective study. J Gynecol Obstet Hum Reprod. 2017;46(8):629-635.
- Bishop LA, Richter KS, Patounakis G, et al. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Schuh-Huerta SM, Johnson NA, Rosen MP, et al. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27(2):594-608.
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. Jama. 2017;318(14):1367-1376.
- Abdalla H, Thum MY. Repeated testing of basal FSH levels has no predictive value for IVF outcome in women with elevated basal FSH. Hum Reprod. 2006;21(1):171-174.
- Broer SL, Dólleman M, Opmeer BC, et al. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011 ;17(1):46-54.
- Jayaprakasan K, Campbell B, Hopkisson J, et al. Establishing the intercycle variability of three-dimensional ultrasonographic predictors of ovarian reserve. Fertil Steril. 2008;90(6):2126-2132.
- Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89(6):1670-1676.
- Jung S, Allen N, Arslan AA, et al. Demographic, lifestyle, and other factors in relation to antimüllerian hormone levels in mostly late premenopausal women. Fertil Steril. 2017;107(4):1012-1022.
- Liu XY, Yang YJ, Tang CL, et al. Elevation of antimüllerian hormone in women with polycystic ovary syndrome undergoing assisted reproduction: effect of insulin. Fertil Steril. 2019;111(1):157-167.
- Penarrubia J, Fábregues F, Manau D, et al. Basal and stimulation day 5 anti-Müllerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist–gonadotropin treatment. Hum Reprod. 2005;20(4):915-922.
- Tal R, Seifer DB, Wantman E, et al. Antimüllerian hormone as a predictor of live birth following assisted reproduction: an analysis of 85,062 fresh and thawed cycles from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System database for 2012–2013. Fertil Steril. 2018;109(2):258-265.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Rabab Awad Farhood, Vian Hussam Almansi Alqani* and Ashwaq Kadhim MohammedCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.