Research - (2025) Volume 20, Issue 1
Subcutaneous wound infiltration with tramadol for post cesarean section pain relief: A double-blinded placebo randomized controlled trial
Nada Alayed1,8, Salwa Neyazi1,8, Ahmed Sherif Abdel Hamid1,2*, Haytham Hagsaad Abdelrahman Mohamed1, Mohamed abdel Razeq1,2, Marawan Mohamed Gamal1,2, Ahmed Reda2,3, Gamal Mustafa Farag2, Halima Al-Dhabyani2, Mohamed Mahmoud Abdel Aziz4, Ahmed Abdel Kader Ahmed3, Asmaa Moatasem Elgharib4,5, Mohammed Abdullah Alatawi6, Amal Ali Fadlallah kalifa7 and Mohamed el Sherbeeny2*Received: 02-Jan-2025, Manuscript No. gpmp-25-157535; Editor assigned: 03-Jan-2025, Pre QC No. P-157535; Reviewed: 23-Jan-2025, QC No. Q-157535; Revised: 30-Jan-2025, Manuscript No. R-157535; Published: 31-Mar-2025
Abstract
Introduction: Cesarean delivery is the most frequently performed surgical procedure globally. Administering analgesia through wound infiltration using narcotics or local anesthetics can lower pain levels and reduce the amount of postoperative analgesics needed.
Objective: To assess the effects of tramadol infiltration at the incision site before skin closure in patients undergoing cesarean delivery on postoperative pain levels and the need for analgesics in comparison to lidocaine.
Methods: This randomized controlled trial was performed at Ain Shams University Maternity Hospital, where 99 pregnant women scheduled for cesarean section delivery were RANDOMLY assigned to receive tramadol, lidocaine, or placebo subcutaneously before the closure of the skin. The primary outcome we measured was pain assessment at 2, 6, 12, and 24 hours post-operation using the visual analog scale (VAS). Secondary outcomes included the average time until the first analgesic request and the total diclofenac consumption within 24 hours.
Results: VAS scores were statistically significantly lower in the Tramadol group when compared to the other two groups at 2 hours (h) (p<0.001), 6 hours (p<0.001), and 12 hours (p<0.01). However, at 24 h, the VAS score in the Tramadol group was significantly lower than that in the Placebo group (p<0.01) but was similar to the Lidocaine group. The difference in VAS scores between the Lidocaine and Placebo groups was not statistically significant. The time to the first analgesic request was significantly prolonged in the Tramadol group: 2.5 ± 0.9 vs. 2.4 ± 0.8 vs. 5.8 ± 3.1 hours in the Placebo, Lidocaine, and Tramadol groups, respectively, p<0.001.
Conclusion: Local wound infiltration with tramadol significantly reduced pain scores, delayed first analgesic request, and lowered 24-hour analgesic consumption. Lidocaine showed no additional benefit over placebo. Tramadol infiltration is effective for post-operative analgesia in cesarean sections.
Keywords
Lidocaine; Post-cesarean section analgesia; Post-operative pain; Tramadol
Introduction
The global prevalence of cesarean sections is on the rise, and pain after surgery continues to be a major issue. It has been demonstrated that regional anesthetic methods in this group can decrease the use of opioids after surgery, enhance patient comfort, and facilitate early mobility and breastfeeding [1].
Pain is recognized as a defense mechanism that responds to different harmful stimuli. The International Association for the Study of Pain characterizes acute pain as "An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” Postoperative pain is the primary form of acute pain, beginning with the trauma from the surgery and continuing until the tissues have healed [2].
Postoperative pain can lead to a range of negative physiological and psychological effects. It can be measured using direct methods such as verbal rating scales, visual analog scales (VAS), numerical rating scales, or the Prince Hennery score. Despite the availability of effective pain therapies, managing postoperative pain remains a challenge, with over half of patients experiencing pain, particularly during the later stages of recovery [3].
Historically, pain management following a cesarean section has relied heavily on opioid medications. However, because opioids can cause a variety of side effects, including severe issues like respiratory depression, when taken systemically, there is a growing trend toward using local anesthetic techniques instead. The PROSPECT (PROcedure SPECific postoperative pain managemenT) group, consisting of specialists in anaesthesiology and surgery, advocates for the use of a fascial plane block, like a Transversus Abdominis Plane (TAP) block or wound infiltration, to manage postoperative pain when intrathecal opioids are not utilized [4].
Subcutaneous wound infiltration with local anesthetics is proposed as a method to decrease postoperative pain. Tramadol engages both opioid and non- opioid mechanisms to alleviate pain and has demonstrated local anesthetic properties on peripheral nerves [5].
Various research studies have shown that using 2% lidocaine wound infiltration led to a reduction in postoperative pain and decreased the need for additional pain relief medication. In another study involving 1% lidocaine, there was no notable difference in postoperative pain or the number of requests for pain relief when compared to a placebo group. Additionally, a different study found that administering subcutaneous pethidine or tramadol after a cesarean section provided significantly better pain relief and reduced the need for morphine compared to long-acting bupivacaine [6-9].
The current work aims to evaluate the impact of tramadol infiltration at the incision site before closure of the skin in patients undergoing cesarean section on post-operative pain and analgesic requirements in comparison to lidocaine.
Materials and Methods
This research is a randomized controlled trial conducted at Ain Shams University Maternity Hospital over six months. All participants provided informed consent following thorough counseling. The study received ethical approval from the Faculty of Medicine, Ain Shams University. All procedures adhered to the Declaration of Helsinki. The research follows the CONSORT guidelines for randomized controlled trials. Written consent was obtained from all participants before randomization. The study has been registered with the Pan African Trials Clinical Registry (PACTR201711002756172).
We enrolled 99 women between the ages of 20 to 35 years, who were at term and scheduled to undergo elective cesarean delivery via a Pfannenstiel incision. Participants with significant medical issues, bleeding disorders, substance abuse, or allergies to the study medication were excluded from the trial.
The primary outcome measured was pain assessment using the Visual Analog Scale (VAS) at 2, 6, 12, and 24 hours post-operation.
Secondary outcomes included the average time until the first analgesic request and the total diclofenac consumption within 24 hours.
Sample size justification: The necessary sample size has been calculated using the Power Analysis and Sample Size software version 08.0.9 (PASS; NCSS, LLC, Kaysville, Utah). It is anticipated that a total sample size of 99 participants, evenly distributed among the three study groups (n=33 per group), would provide an 80% power to identify a statistically significant difference in pain scores at 6 hours post-surgery through a two-sided F test, with the type I error rate set at a two-sided value of 0.05 (confidence level of 95%). The expected mean (SD) pain scores are projected to be 3.78 (1.76), 3.83 (1.08), or 4.53 (1.59) for the lidocaine [10], tramadol [11] or placebo group (10), respectively. The variation in the means is indicated by their standard deviation, estimated at 0.34. The assumed common standard deviation within a group is 1.08.
Randomization, allocation, and masking: Ninety-nine cases were included in the study and were randomly assigned into three equal groups, each consisting of 33 patients, using simple randomization from a computer-generated randomization table. In the first placebo group A (n=33 women), the wound was infiltrated with 20 ml of 0.9% saline during skin closure. Based on the visual analog scale, patients received diclofenac sodium 75mg IM as needed. In the second group B (n=33 women): The wound was infiltrated with 20 ml of 1% lidocaine hydrochloride during skin closure. Similar to group A, patients received diclofenac sodium 75mg IM on demand according to the visual analog scale. In the third group, C (n=33 women), the wound was infiltrated with 50mg of Tramadol diluted in 20 ml of 0.9% saline during skin closure. Based on the visual analog scale, patients also received diclofenac sodium 75mg IM as needed. The nurse coordinator, who had no interaction with the participating investigators, conducted the randomization. The medications' names and numbers were distributed in sealed opaque envelopes, and the anesthetists prepared the medications only after unsealing the envelopes immediately before use, ensuring that the obstetricians remained unaware of the medication employed. All patients received the medications following the cesarean section and prior to the skin closure.
Procedure: All patients provided written informed consent after the study explanation. A comprehensive history was taken, including maternal age, gravidity, and previous obstetric history. A thorough general examination focused on vital signs (blood pressure, pulse, temperature), chest and heart assessment, and abdominal examination for gestational age, fetal weight, amniotic fluid amount, fetal position, heart sounds, uterine contractions, and any surgical scars. Routine laboratory tests included a complete blood count (CBC), liver and kidney function tests, prothrombin time (PT), and concentration (PC). A trans-abdominal ultrasound assessed gestational age, placental implantation, and fetal weight.
Pain assessment was conducted using the VAS at 2, 4, 6, 12, and 24 hours following surgery. The Visual Analog Scale (VAS), which measures pain on a 10- point scale, is the gold standard for pain assessment in research settings. This method includes a 100mm straight line, where one end indicates no pain and the opposite end signifies the most extreme pain imaginable. Patients were instructed to mark their perceived pain level on this line. Following the surgery, patients were administered postoperative analgesia with Diclofenac sodium 75mg (Voltaren, Novartis- Switzerland) via intramuscular injection as needed.
Statistical analysis: Data were collected, tabulated, and analyzed on a personal computer using IBM© SPSS© Statistics version 21 (IBM© Corp., Armonk, NY). The Kolmogorov-Smirnov goodness of fit test was employed to assess the normality of numerical data distribution. Normally distributed numerical data were presented as mean and standard deviation (SD), and differences between the two groups were compared using one-way analysis of variance (ANOVA). Qualitative data were presented as counts and percentages, with the chi-square or Fisher’s exact test applied to compare the three groups when appropriate. All P values were two- tailed, and P<0.05 was considered statistically significant.
Results
Fig. 1. show the consort flow chart of the participants patients. The general characteristics, obstetric history, and past medical and surgical backgrounds of the women included in the study were similar across the Placebo, Lidocaine, and Tramadol groups. As illustrated in Tab. 1., there was no statistically significant difference among the three groups regarding age (p=0.795), body mass index (BMI) (p=0.164), and gestational age at delivery (p=0.698).
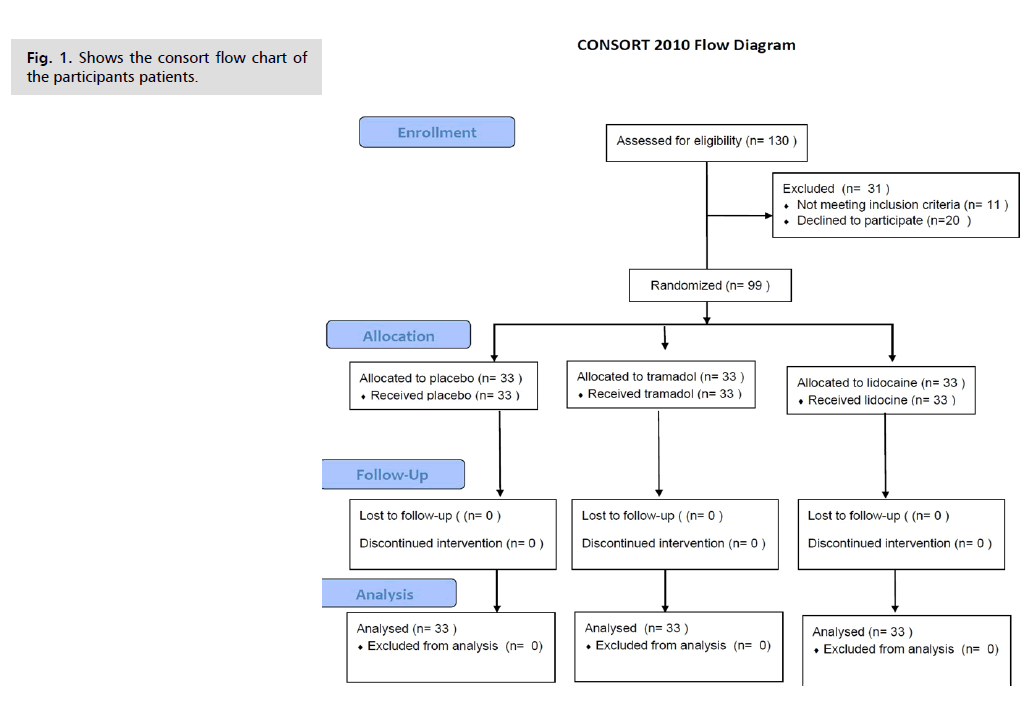
Fig. 1. Shows the consort flow chart of the participants patients.
| Variables | Placebo (n=33) | Lidocaine (n=33) | Tramadol (n=33) | p-value |
|---|---|---|---|---|
| Age (years) | 30.2 ± 4.3 | 29.5 ± 4.4 | 29.9 ± 3.9 | 0.795 |
| BMI (kg/m2) | 28.1 ± 3.9 | 26.5 ± 3.8 | 27.8 ± 3.1 | 0.164 |
| Gestational age (weeks) | 38.5 ± 1.6 | 38.4 ± 1.8 | 38.7 ± 1.5 | 0.698 |
Tab. 1. Patients’ characteristics.
In the Placebo, Lidocaine, and Tramadol groups, five women (15.2%), six women (18.2%), and four women (12.4%) were primigravid, respectively (p=0.772). A history of one or more previous cesarean sections was present in 21 women (63.6%), 19 women (57.7%), and 21 women (63.6%) in the Placebo, Lidocaine, and Tramadol groups, respectively (p=0.744) [data not tabulated].
We observed that the VAS scores were significantly lower in the Tramadol group compared to the other two groups at 2 hours (h) (p<0.001), 6 hours (p<0.001), and 12 hours (p<0.01). At 24 h, the VAS score in the Tramadol group was significantly lower compared to the Placebo group (p<0.01) but comparable to that of the Lidocaine group (Tab. 2.). The difference in VAS scores between the Lidocaine and Placebo groups was insignificant (Tab. 2.).
| Visual analog scale | Placebo (n=33) | Lidocaine (n=33) | Tramadol (n=33) | p- value |
|---|---|---|---|---|
| VAS at 2 h | 4.8 ± 2.2 | 5.1 ± 2.1 | 2.2 ± 1.8* | <0.001 |
| VAS at 4 h | 3.4 ± 1.8 | 3.2 ± 1.7 | 2.2 ± 0.5 | 0.415 |
| VAS at 6 h | 3.0 ± 1.2 | 3.2 ± 1.0 | 2.2 ± 0.8* | <0.001 |
| VAS at 12 h | 4.8 ± 1.5 | 4.8 ± 1.1 | 3.6 ± 1.8† | <0.01 |
| VAS at 24 h | 4.1 ± 0.8 | 3.6 ± 0.9 | 3.0 ± 1.3‡ | <0.001 |
| TFA request, h | 2.5 ± 0.9 | 2.4 ± 0.8 | 5.8 ± 3.1* | <0.001 |
Tab. 2. Comparison between the studied groups as regards pain scores and time to first analgesic request.
Additionally, we found that the time until the first analgesic request was significantly longer in the Tramadol group compared to the other two groups; 2.5 ± 0.9 vs. 2.4 ± 0.8 vs. 5.8 ± 3.1 hours in the Placebo, Lidocaine, and Tramadol groups, respectively, p<0.001 (Tab. 2., Fig. 2.). However, there was no statistically significant difference in the meantime to the first analgesic request between the Lidocaine and Placebo groups.

Fig. 2. Mean time to first analgesic request in the three study groups. Error bars represent the standard error of the mean.
Data presented as mean ± standard deviation, analysis done using one-way analysis of variance (ANOVA), VAS=visual analog scale, h=hour, TFA=time to first analgesic request, *P<0.001 vs. Placebo group & Lidocaine group, †P<0.01 vs. Placebo group & Lidocaine group, ‡P<0.001 vs. Placebo group only.
When comparing postoperative analgesic consumption among the three groups, the number of patients needing diclofenac at two h was significantly fewer in the Tramadol group (5 women [15.2%]) compared to the Placebo group (21 women [63.6%]) and the Lidocaine group (25 women [75.8%]). There were also significantly fewer patients needing diclofenac at 24 h in the Tramadol group (16 women [48.5%]) when compared to the Placebo and Lidocaine groups (31 women [93.9%] and 28 women [84.8%], respectively). The number of women requiring analgesia at 4 h, 6 h, and 12 h was similar across the three study groups (Tab. 3.).
| Diclofenac consumption | Placebo (n=33) | Lidocaine (n=33) | Tramadol (n=33) | p-value |
|---|---|---|---|---|
| at 2 h | 21 (63.6) | 25 (75.8) | 5 (15.2) | <0.001 |
| at 4 h | 10 (30.3) | 6 (18.2) | 7 (21.2) | 0.479 |
| at 6 h | 2 (6.1) | 3 (9.1) | 7 (21.2) | 0.223 |
| at 12 h | 28 (84.8) | 32 (97) | 26 (78.8) | 0.113 |
| at 24 h | 31 (93.9) | 28 (84.8) | 16 (48.5) | <0.001 |
Tab. 3. Comparison of analgesic consumption between the study groups.
Furthermore, the cumulative 24-hour consumption of diclofenac was significantly lower in the Tramadol group relative to the other two groups (Fig. 3.). However, the number of patients requiring pethidine was similar across all groups: 3 women (9.1%) vs. two women (6.1%) vs. one woman (3%) in the Placebo vs. Lidocaine vs. Tramadol groups, respectively (p=0.869).

Fig. 3. Cumulative 24-hour diclofenac consumption in the three study groups.
Discussion
Tramadol has demonstrated a local anesthetic effect in humans, similar to prilocaine, despite initially being thought to work primarily through spinal and supraspinal mechanisms. Studies show that intradermal tramadol can provide local anesthesia and significantly reduce postoperative pain compared to lidocaine in minor surgeries [1].
Our results and their comparison to similar studies
The present research indicated that VAS scores were notably lower with subcutaneous administration of tramadol compared to lidocaine and placebo at 2, 6, 12, and 24 hours. The duration until the initial request for analgesics was significantly extended, and the total amount of analgesic consumed over 24 hours was significantly diminished with tramadol.
These results differ from the study conducted by Ghenaee and colleagues, which involved 100 participants randomly assigned to receive lidocaine 2% (4 mg/kg diluted in 30 mL of normal saline). They determined that administering lidocaine 2% into the wound of a cesarean section incision alleviated postoperative pain and reduced the requirement for further analgesics [5].
The results we found were supported by Kessous, et al. in their Randomized Controlled Trial (RCT), which investigated the administration of a 1% lidocaine solution at the incision site during cesarean sections. They determined that there was no significant difference in postoperative pain levels or requests for analgesics between the groups receiving lidocaine and those given a placebo [6].
In our research, tramadol wound infiltration was found to be more effective
than lidocaine, as shown in a study by Jabalameli, et al. [7] the study involved 120 patients undergoing cesarean sections, randomized into four groups: Pethidine (Group P), Tramadol (Group T), Bupivacaine (Group B), and a control group (Group C). Pain intensity and opioid consumption were assessed postoperatively. The results showed significantly lower VAS scores in groups T and P compared to B and C, especially in the initial hours after surgery. Additionally, tramadol and pethidine required less additional analgesia, likely due to tramadol's prolonged action.
In a study by Sachidananda, et al., tramadol enhanced the effects of bupivacaine, resulting in an extended pain- free period and reduced the need for additional analgesia [9].
The results of the current research opposed those found by Jayashree and colleagues. Their study included 60 women who had cesarean deliveries using spinal anesthesia. They evaluated tramadol against bupivacaine and found that bupivacaine provided better analgesic properties than tramadol. Their research indicated that tramadol had a notable pain-relieving effect and a longer duration of action [12].
Clinical implication of our study
The data showed a high incidence of postoperative pain affecting mothers, families, medical professionals, and healthcare services. Our findings indicate that local tramadol administration in the surgical wound significantly lowered pain levels, delayed the first request for pain relief, and decreased overall pain medication consumption within 24 hours. We encourage tramadol infiltrating the wound for postoperative pain management after cesarean sections.
Strengths and Limitations of the Study
The current research benefits from being a randomized controlled trial, which helps minimize selection bias, particularly through the blinding process. Our study possesses sufficient power. Conversely, the limitations of our research include the absence of patient satisfaction measurements, a lack of long-term follow-up, a restricted pain scale, and the absence of blinding for the anesthesiologist, despite their non-involvement in data collection.
Recommendations for future study
Further investigation is needed to evaluate the effects of bupivacaine and various opioid injections on incisions from cesarean sections.
Conclusion
Local wound infiltration with tramadol significantly reduced pain scores, delayed first analgesic request, and lowered 24-hour analgesic consumption. Lidocaine showed no additional benefit over placebo. Tramadol infiltration is effective for post-operative analgesia in cesarean sections.
Funding
This research received no external funding.
Disclosure of Interest
The authors declare no conflict of interest.
Ethics Approval and Informed Consent to Participate
Following local regulations, the study gained ethical committee approval from the Faculty of Medicine, AIN University. All procedures were done per the Declaration of Helsinki.
Data Sharing
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgment
Not applicable.
References
- Grape S, Kirkham KR, Albrecht E. Transversus abdominis plane block versus local anaesthetic wound infiltration for analgesia after caesarean section: A systematic review and meta-analysis with trial sequential analysis. Eur J Anaesthesiol. 2022;39(3):244-51.
- Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976-1982.
- Muñoz-Leyva F, El-Boghdadly K, Chan V. Is the minimal clinically important difference (MCID) in acute pain a good measure of analgesic efficacy in regional anesthesia?. Reg Anesth Pain Med. 2020;45(12):1000-1005.
- Roofthooft E, Joshi GP, Rawal N, et al. PROSPECT guideline for elective caesarean section: updated systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76(5):665-680.
- Ghenaee MM, Rahmani S, Jafarabadi MI. Local lidocaine 2% in postoperative pain management in cesarean delivery. J Family Reprod Health. 2015;9(1):19.
- Kessous R, Wiznitzer A, Polachek H, et al. Preoperative analgesia with local lidocaine infiltration for post cesarean delivery pain management. J Matern Fetal Neonatal Med. 2012;25(7):1131-1134.
- Jabalameli M, Hazegh P, Talakoub R. Preemptive subcutaneous tramadol for post-operative pain in lower abdomen surgeries: a randomized double blinded placebo-control study. Adv Biomed Res. 2013;2(1):68.
- Jabalameli M, Safavi M, Honarmand A, et al. The comparison of intraincisional injection tramadol, pethidine and bupivacaine on postcesarean section pain relief under spinal anesthesia. Adv Biomed Res. 2012;1(1):53.
- Sachidananda R, Joshi V, Shaikh SI, et al. Comparison of analgesic efficacy of wound infiltration with bupivacaine versus mixture of bupivacaine and tramadol for postoperative pain relief in caesarean section under spinal anaesthesia: A double-blind randomized trial. J Obstet Anaesth Crit Care. 2017;7(2):85-89.
- Navali N, Fouladi RF, Nikpour MA. A comparison of post-incisional subcutaneous, intramuscular, and subcutaneous plus intramuscular infiltrations of lidocaine in postcaesarean section pain control. S Afr J OG. 2013;19(1).
- Behdad S, Sekhavat L, Ayatollahi V, et al. Comparison of postoperative analgesic effect of tramadol and bupivacaine subcutaneous infiltration in patients undergoing cesarean section. Acta clinica Croatica. 2013;52(1):93-97.
- Jayashree V, Latha K, Dhakshinamoorthy M, et al. Intraincisional injection of tramadol versus bupivacaine in post-caesarean pain relief. Int J Clin Obstet. 2019;3:355-360.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Nada Alayed1,8, Salwa Neyazi1,8, Ahmed Sherif Abdel Hamid1,2*, Haytham Hagsaad Abdelrahman Mohamed1, Mohamed abdel Razeq1,2, Marawan Mohamed Gamal1,2, Ahmed Reda2,3, Gamal Mustafa Farag2, Halima Al-Dhabyani2, Mohamed Mahmoud Abdel Aziz4, Ahmed Abdel Kader Ahmed3, Asmaa Moatasem Elgharib4,5, Mohammed Abdullah Alatawi6, Amal Ali Fadlallah kalifa7 and Mohamed el Sherbeeny2*2Department of Obstetrics and Gynecology, Faculty of Medicine, Ain-Shams university, Cairo, Egypt
3Department of Obstetrics and gynecology, Badr University Cairo (BUC), Cairo, Egypt
4Department of Anaesthesia, Faculty of Medicine, King saud university, Riyadh, Saudi Arabia
5Department of Anaesthesia, Faculty of Medicine, Assiut University, Assiut, Egypt
6Department of Obstetrics and Gynecology, Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia
7Department of Obstetrics and Gynecology, Faculty of Medicine, Elimam Elmahdi University, Khartoum, Sudan
8The first and second authors are contributed equally to this research, Saudi Arabia
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.