Research - (2025) Volume 20, Issue 1
Serum irisin level in normotensive versus preeclamptic pregnant women
Amal Muner Mubarak*, Aseel Abdulameer Mohammed and Batool AlkhalidiReceived: 04-Feb-2025, Manuscript No. gpmp-25-161480; Editor assigned: 06-Feb-2025, Pre QC No. P-161480; Reviewed: 20-Feb-2025, QC No. Q-161480; Revised: 28-Feb-2025, Manuscript No. R-161480; Published: 31-Mar-2025
Abstract
Introduction: Hypertensive disorders are a common complication of pregnancy that put women and their fetuses at disproportionate risk for further complications, as well as life-long sequelae. Irisin was reported to have vasodilator, antioxidant, anti-inflammatory effects and to improve endothelial dysfunction, which suggest that it may aid in improvement of placental ischemia during preeclampsia.
Aim of the study: To assess level serum irisin in normotensive and preeclamptic pregnancies.
Patients and method: A case control study, conducted at the Department of Obstetrics and Gynecology at Al-Zahra’a Teaching Hospital, Al-Najaf city, from the 1st of September 2021 to the first of July 2022. In the current study 60 pregnant women were enrolled, and divided into 2 groups: Thirty pregnant with preeclampsia from 28-40 weeks as case group and other thirty pregnant as healthy uncomplicated normotensive pregnancies as control group.
Results: Serum irisin level was (7.28 ± 1.3) in patient compared to (9.2 ± 1.37) in control group with a (P<0.001). The validity of the test to detect the preeclampsia show that sensitivity was 93%, 54% specificity, negative predictive value was 90.4%, positive predictive value was 56% and accuracy was 70% at 90% CI (0.634 -0.873) with significant association.
Conclusions: Serum irisin level was decreased significantly in women with preeclampsia than that in normal pregnancy.
Keywords
Preeclampsia; Irisin
Introduction
Hypertensive disorders are a common complication of pregnancy that place women and their fetuses at disproportionate risk for further complications, as well as life-long sequelae [1].
More than 10% of women will develop pre-eclampsia in their first pregnancy and although the vast majority of these will have successful pregnancy outcomes, the condition can give rise to severe multisystem complications including cerebral hemorrhage, renal dysfunction, hepatic dysfunction and respiratory compromise. The pathophysiology of preeclampsia has not yet been fully clarified. The prevailing theory is that impaired transformation of the spiral arteries results in subsequent relative placental ischemia, causing the release of vasoactive factors into the circulation, which then give rise to endothelial-mediated end-organ damage and the clinical manifestations of the disease [2].
Numerous studies have highlighted the beneficial and protective properties of irisin as a vasodilator, antioxidant, and anti-inflammatory agent, as well as its ability to improve endothelial dysfunction. These findings suggest that irisin may aid in the improvement of placental ischemia during preeclampsia [3-9]. However, there are contradictory reports regarding the serum profile of irisin in preeclamptic pregnancies.
The aim of the study
To assess serum irisin level in normotensive and preeclamptic pregnancies.
Study design and setting
A case control study was conducted in Iraq-najaf city at Al-Zahra’a Teaching Hospital, Department of Obstetrics and Gynecology. The study protocol was approved by the local medical research ethics committee of Al-Zahra’a Teaching Hospital, Department of Obstetrics and Gynecology. The purpose and procedures were explained to all participants and they were given the right to participate or not, informed consent was taken from all participants with reassurance that the information will be kept confidentially.
The current study included 60 Iraqi pregnant women in the third trimester of singleton pregnancies with living fetuses, with gestational ages ranging from 28 to 40 weeks. Gestational age was determined based on the last menstrual period and confirmed through an early ultrasound performed during the first trimester.
The participants were divided into two groups: the control group consisted of 30 women with singleton, normotensive, uncomplicated pregnancies attending routine antenatal visits or admitted for elective cesarean delivery, while the study group comprised 30 women diagnosed with preeclampsia upon admission. Both groups were matched in terms of maternal age, Body Mass Index (BMI) calculated using the standard formula (kg/m²) from antenatal records, gestational age, and parity.
Women with diabetes mellitus, chronic liver or renal disease, cancer, chronic cardiac disease, active labor, infections, multiple pregnancies, intrauterine growth restriction, intrauterine death, or fetal microsomia were excluded from the study.
Pre-eclampsia is defined as hypertension of at least 140/90 mmHg documented on at least two separate occasions and at least 4 hours apart and in the presence of at least 300 mg protein in a 24-hour collection of urine, arising de novo after the 20th week of pregnancy in a previously normotensive woman and resolving completely by the sixth postpartum week [1].
Method
A volume of seven ml of fasting venous serum sample was collected from all participants, all samples were stored at room temperature for at least one hour to allow the blood to clot followed by centrifugation for 20 minutes at 1000 x g at 2-8 ℃ and the content of tube has been divided into 2 tubes:
1. Five ml of the collected blood was sent for general investigations in the hospital laboratory,
2. The other 2 ml were used for determination of Irisin levels in a private laboratory. Serum specimens were aliquoted and stored at –80 ℃ until Irisin levels were analyzed by ELISA (Enzyme-Linked Immunosorbent Assay) in private laboratory. The kit name was: Human (Irisin) ELISA kit catalog number: E-EL-H612. Lot: RK4GZX8HJC. And the instrument data: ELx800 bio Elisa Reader, SN:193580 USA.
Statistical analysis
The collected data proceed for statistical analysis via SPSS program in a new version (V25) by independent t test and correlation test and the data presented in form of Tables and Figures.
P value at a set of ≤ 0.05 were considered significant in all statistical analysis. ROC was completed to measure the validity of the Irisin test to detect the preeclampsia by measuring of (sensitivity, specificity, NPV, PPV and the accuracy).
Results
In the current study sixty patients were enrolled, half of them as studies group and the other half as control group with age range between (16-40) years and mean age of the studied groups was (24.9 ± 6.89) years, mean BMI was (27.03 ± 3.79) kg/m2, mean gestational age was (35.15 ± 3.2) weeks, and mean of parity was (1.71 ± 1.48) (Tab. 1.).
| Parameters | Mean | SD |
|---|---|---|
| Age (years) | 24.9 | 6.89 |
| BMI (kg/m2) | 27.03 | 3.79 |
| GA (weeks) | 35.15 | 3.2 |
| Parity | 1.71 | 1.48 |
Tab. 1. Means of patient’s criteria in the study group.
As shown in Tab. 2., the main age group in studies group was between 21-30 years (43.3%) while for control group was in ≤ 20 years (36.7%). The main group of gestational age in studies group was in 31 weeks and more (86.6%) while it is in >35 weeks in control group (63.3%). BMI was more common in overweight group for both groups of the study (43.3%) in case and (40.0%) in control group. Multipara was common in both groups (53.3%) in studies group and (70.0%) in control group.
| Case (no.=30) | Control (no.=30) | ||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (years) | ≤ 20 | 10 | 33.3 | 11 | 36.7 |
| 21-30 | 13 | 43.3 | 10 | 33.3 | |
| 31-40 | 7 | 23.4 | 9 | 30.0 | |
| GA (weeks) | 28-30 | 4 | 13.4 | 3 | 10.0 |
| 31-35 | 13 | 43.3 | 8 | 26.7 | |
| 36-40 | 13 | 43.3 | 19 | 63.3 | |
| BMI (kg/m2) | Normal (18.5-24.9) | 9 | 30.0 | 10 | 33.3 |
| Overweight (25-29.9) | 13 | 43.3 | 12 | 40.0 | |
| Obese (≥ 30) | 8 | 26.7 | 8 | 26.7 | |
| Parity | Primi | 14 | 46.7 | 9 | 30.0 |
| Multipara | 16 | 53.3 | 21 | 70.0 | |
Tab. 2. Distribution of patients in the studied groups.
Tab. 3. shows that there are no significant differences between case and control groups regarding; age, BMI, GA, and parity (P ≥ 0.05).
| Parameters | Means ± SD | P-value | |
|---|---|---|---|
| Case (no.=30) | Control (no.=30) | ||
| Age (years) | 24.6 ± 6.44 | 25.23 ± 7.42 | 0.7 Ns |
| BMI (kg/m2) | 26.83 ± 3.26 | 27.23 ± 4.3 | 0. 68 Ns |
| GA (weeks) | 34.56 ± 2.71 | 35.7 ± 3.58 | 0.1 Ns |
| Parity | 1.56 ± 1.33 | 1.86 ± 1.63 | 0.4 Ns |
Tab. 3. Differences between demographic criteria in the studied groups.
Tab. 4. and Fig. 1. showed that there is highly significant increase in systolic and diastolic blood pressure in case group than that in control group (P<0.001).
| Parameters | Means ± SD | P-value | |
|---|---|---|---|
| Case (no.=30) | Control (no.=30) | ||
| Systolic BP | 159.3 ± 8.27 | 115.0 ± 7.3 | <0.001 Hs |
| Diastolic BP | 103.0 ± 4.66 | 75.6 ± 5.68 | <0.001 Hs |
Tab. 4. Differences between blood pressure in the studied groups.
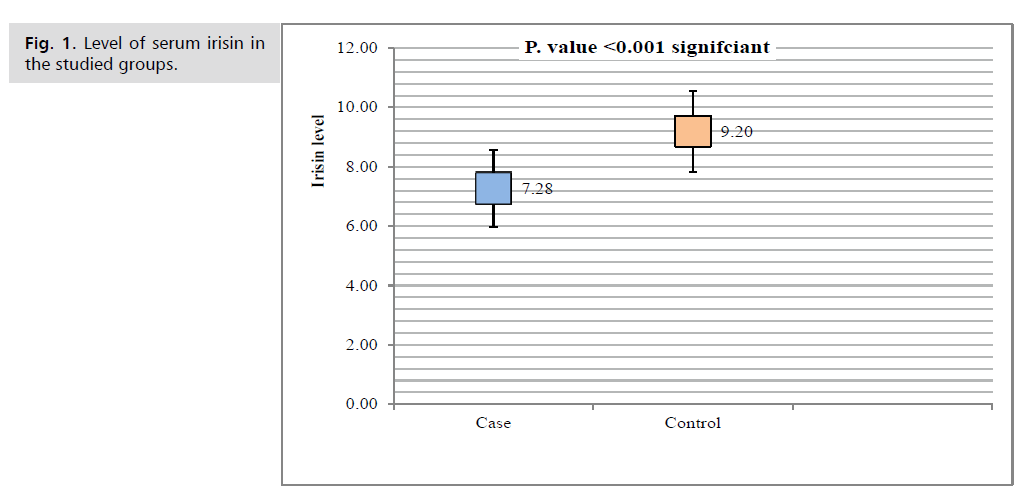
Fig. 1. Level of serum irisin in the studied groups.
Tab. 5. and Fig. 1. showed that serum irisin level was (7.28 ± 1.3) in case group while it was (9.2 ± 1.37) in control group. There is highly significantly decreased in case group than that in control group (P<0.001).
| Parameters | Case (no.=30) | Control (no.=30) | P-value |
|---|---|---|---|
| S. Irisin (Means ± SD), range | 7.28 ± 1.3, (5.37-10.3) | 9.2 ± 1.37, (6.099-18.02) | <0.001 Hs |
Tab. 5. Difference between serum Irisin level in the studied groups.
Receiver Operator Characteristic (ROC) curve was used to find the cutoff value of irisin to correlation of the preeclampsia with better accuracy.
As shown in (Fig. 2. and Tab. 6.), the characteristics of the ROC curve find that at area under the curve (0.892) the sensitivity was 93%, 54% specificity, Negative Predictive Value (NPV) was 90.4%, Positive Predictive Value (PPV) was 56% and accuracy was 70% at 90% CI (0.634 -0.873) with significant association (P=0.004).
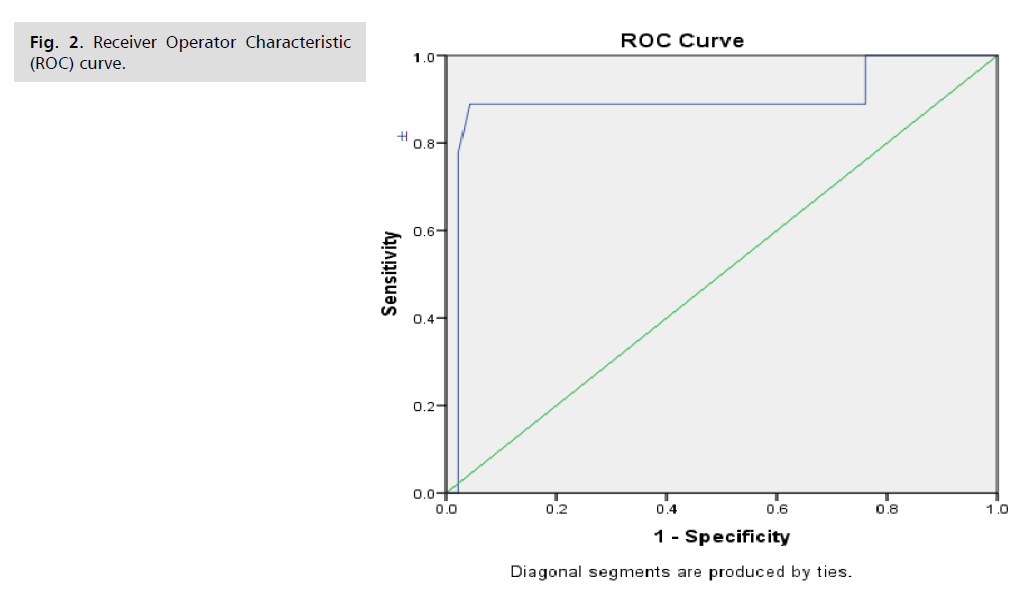
Fig. 2. Receiver Operator Characteristic (ROC) curve.
| Characteristic | Result |
|---|---|
| Cutoff value of irisin | ≤ 8.9 |
| Area Under the Curve (AUC) | 0.892 |
| 95 % Confidence Interval (CI) | 0.634 -0.873 |
| P value | <0.001 HS |
| Sensitivity % | 93 % |
| Specificity % | 54 % |
| Positive Predictive Value (PPV %) | 56.2 % |
| Negative Predictive Value (NPV %) | 90.4 % |
| Accuracy of the test % | 70.0 % |
Tab. 6. Characteristics of the ROC curve.
Discussion
Preeclampsia is a multisystem progressive disorder and among the most frequent causes of maternal and neonatal death. Women with PE are at high risk to develop chronic kidney disease, cardiovascular disease, liver failure, coagulopathy and dysfunction of vital organs, and without treatment it can cause convulsions (eclampsia), intracranial hemorrhage, multiple organ failure and other serious complications and death [10-12], additionally, PE has serious adverse effect on the fetus and neonate [13].
The exact causes of PE are not exactly known to date, however Systemic vascular dysfunction has been proposed as an essential pathogenic origin of the disease (preeclampsia). This dysfunction raises the sensitivity of the vasculature to vasoactive substances, with a consequent decrease in perfusion and loss of fluid from the intravascular compartment. So, both of hemodynamic variations, and the stimulation of the coagulation cascade with the formation of microthrombi secondary to endothelial damage, will produce the different clinical complications associated with preeclampsia. Several potential biochemical markers have been proposed in the diagnosis of hypertensive syndrome of pregnancy [14-18].
The physiological roles of serum irisin are still under examination many researchers pay special attention for several sources of irisin n pregnancy and their effects on the placenta to understand its roles in maternal and feto-placental metabolism and disease [19].
Garcés M, et al. study concluded that irisin’s precursor FNDC5 is found in the tissues of placenta including syncytiotrophoblast and cytotrophoblast cells of normal pregnancies. This may suggest that in preeclampsia, altered serum irisin levels may be related to the abnormal placentation and/or placental insufficiency. Some studies reported lower irisin levels with increased weight, others reported higher levels in obese individuals [20,21].
The most important finding in the current study showed that serum irisin level was decreased in studies group than that in control group with highly significant difference between the groups (P<0.001). which is same that revealed by Vivek K, et al, in a meta-analysis study revealed that the serum irisin maternal level was decreased significantly in preeclampsia patients as compared to normotensive pregnant women [22].
Also , it is in agreement with Hou, et al. and Turkish study carried by Ozel, A et al., revealed that this decrease of serum irisin level in preeclamptic women may be due to pathologic process, as generalized endothelial disfunction is observed as a major role in the pathophysiology of maternal preeclamptic syndrome [23,24].
Also, it is same that found in Egyptian study carried by Foda AA and Foda EA, in which serum levels of maternal irisin were significantly higher in normal early pregnancies than in mild preeclampsia, and they concluded that delivery is a powerful provocation to the irisin release in circulation of both maternal and fetus [25].
Also, our study is in agreement with Graces MF, et al., who revealed that circulating irisin levels were lower in pregnancies complicated by preeclampsia compared to normal healthy pregnancies in the third trimester [3].
In contrast and Iraqi study carried by Farhan FS, et al. 2018, in a study to investigate the difference in serum irisin levels in normotensive group and preeclamptic groups found that in spite of decreased level of serum maternal irisin during the third trimester of pregnancy in preeclamptic patients than that in healthy control pregnant women but there is no significant difference between both groups were found [26].
Our study was not in agreement with Zhang, et al. 2017, which revealed that there was no significant difference of serum irisin levels between severe pre-eclamptic patients, mild pre-eclamptic patients, and healthy patients as control group [4]. This difference may be attributed to the differences in sample size collection between the studies.
Conclusion
Serum irisin level was decreased significantly in women with preeclampsia than that in normal pregnancy.
Recommendations
1. Larger sample size with more period of time were recommended for further future study to evaluate the cut off value of serum irisin.
2. The result of this study open large window of opportunity for experimental research that irisin may be promising prevention for preeclampsia.
References
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: Pathophysiology, challenges and perspectives. Circ Res. 2019;124(7):1094-1112.
- Waugh JJ, Smith MC. Hypertensive disorders. Dewhurst's Textbook of Obstetrics & Gynaecology. 2012:99-110.
- Garcés MF, Peralta JJ, Ruiz-Linares CE, et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia. J Clin Endocrinol Metab. 2014;99(6):2113-2119.
- Zhang LJ, Xie Q, Tang CS, et al. Expressions of irisin and urotensin II and their relationships with blood pressure in patients with preeclampsia. Clin Exp Hypertens. 2017;39(5):460-467.
- Han F, Zhang S, Hou N, et al. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309(9):H1501-508.
- Lu J, Xiang G, Liu M, et al. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis. 2015;243(2):438-448.
- Zhu DI, Wang H, Zhang J, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138-147.
- Li DJ, Li YH, Yuan HB, et al. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metab. 2017;68:31-42.
- Ma C, Ding H, Deng Y, et al. Irisin: A new code uncover the relationship of skeletal muscle and cardiovascular health during exercise. Front Physiol. 2021;12:620608.
- Kattah A. Preeclampsia and kidney disease: Deciphering cause and effect. Curr Hypertens Rep. 2020;22(11):91.
- Cornelis T, Odutayo A, Keunen J, et al. The kidney in normal pregnancy and preeclampsia. Semin Nephrol. WB Saunders. 2011;31(1):4-14.
- Pankiewicz K, Szczerba E, Maciejewski T, et al. Non-obstetric complications in preeclampsia. Menopause Rev Menopauzalny. 2019;18(2):99-109.
- Weitzner O, Yagur Y, Weissbach T, et al. Preeclampsia: risk factors and neonatal outcomes associated with early-vs. late-onset diseases. J Matern Neonatal Med. 2020;33(5):780-784.
- Han C, Han L, Huang P, et al. Syncytiotrophoblast-derived extracellular vesicles in pathophysiology of preeclampsia. Front Physiol. 2019;10:1236.
- Matsubara K, Matsubara Y, Uchikura Y, et al. Pathophysiology of preeclampsia: The role of exosomes. Int J Mol Sci. 2021;22(5):2572.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022;226(2):S1019-S1034.
- Hannan NJ, Binder NK, Beard S, et al. Melatonin enhances antioxidant molecules in the placenta, reduces secretion of soluble fms-like tyrosine kinase 1 (sFLT) from primary trophoblast but does not rescue endothelial dysfunction: An evaluation of its potential to treat preeclampsia. PLoS One. 2018;13(4):e0187082.
- Roy PK, Sinha S, Nath S, et al. Dysregulation of H2S Level in Plasma and Alteration of Biochemical Parameters in Preeclampsia: A Hospital Based Study.
- Kohan-Ghadr HR, Armistead B, Berg M, et al. Irisin protects the human placenta from oxidative stress and apoptosis via activation of the Akt signaling pathway. Int J Mol Sci. 2021;22(20):11229.
- Fukushima Y, Kurose S, Shinno H, et al. Relationships between serum irisin levels and metabolic parameters in Japanese patients with obesity. Obes Sci Pract. 2016;2(2):203-209.
- Liu BW, Yin FZ, Qi XM, et al. The levels of serum irisin as a predictor of insulin resistance in han chinese adults with metabolically healthy obesity. Clin Lab. 2017;63(5):881-886.
- Vivek K, Thangappazham B, Vykunta A, et al. Maternal serum irisin levels in normotensive and preeclamptic pregnancies: A systematic review and meta-analysis. Gynaecol Endocrinol. 2022;38(4):288-295.
- Ozel A, Davutoglu EA, Firat A, et al. Maternal serum irisin levels in early and late-onset pre-eclamptic and healthy pregnancies. J Obstet Gynaecol. 2018;38(5):642-646.
- Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin. Endocrinol. 2015;83(3):339-343.
- Foda AA, Foda EA. Effects of delivery on maternal & neonatal irisin levels in normal and preeclamptic pregnant women. Pregnancy Hypertens. 2017;10:226-229.
- Farhan FS, Findakly SB, Sersam LW. Serum irisin levels in normotensive and preeclamptic pregnancies. Mustansiriya Med J. 2018;17(2):80-84.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Amal Muner Mubarak*, Aseel Abdulameer Mohammed and Batool AlkhalidiCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.