Research - (2022) Volume 0, Issue 0
Renalase gene polymorphism (rs2576178 and rs10887800) in non severe and severe pre-eclamptic egyptian females
Shimaa Sabry Abd El Ghany1*, Nermine Helmy Mahmoud1, Azza Abd El Rahman Saab1, Mohamed Ahmed Hassan El Kadi2 and Doaa Mostafa Awad1Received: 10-Aug-2022, Manuscript No. gpmp-22-71670; Editor assigned: 11-Aug-2022, Pre QC No. P-71670; Reviewed: 28-Aug-2022, QC No. Q-71670; Revised: 16-Sep-2022, Manuscript No. R-71670; Published: 29-Sep-2022
Abstract
Background: Pre-eclampsia (PE) is a fatal disease for both mother and fetus diagnosed by criteria of ACOG 2017. Gene polymorphisms as renalase gene rs10887800 and rs2576178 are proposed for early prediction and prognosis of PE. This study aimed at assessing the relation between renalase gene polymorphism and development of PE as well as the severity of the disease.
Subjects and Methods: This case-control study included 40females (group I) with PE further subdivided into group Ia 20 non severe PE, group Ib 20 severe PE and group II 20 healthy controls. Renalase gene polymorphisms assessed by PCR-RFLP and confirmed by gene sequencing.
Results: Our results found Renalase rs10887800 homozygous GG& heterozygous AG genotype in 67.5% of PE females compared with 20% in controls, while AA(wild genotype) was found in 32.5 % of PE females and in 80% of controls. A highly significant statistical difference was seen rs10887800 genotype distribution (Χ2 = 12.047; p =0.001) and odds ratio was 8.308 with 95% confidence interval (2.31 - 29.88).Similarly, rs2576178 homozygous GG& heterozygous AG genotype were found in 72.5% of PE females compared to 15% in controls, while AA wild genotype was found in 27.5 % of PE females and in 85% in controls. A highly significant difference found in rs2576178 genotype distribution (Χ2 = 17.712; p = 0.001), the odds ratio was 14.94 with 95% CI (3.65 – 61.19). An increased frequency of G allele of both rs10887800 and rs2576178 was observed in PE females compared to controls. Results of the study were confirmed by Gene sequencing.
Conclusions: Our study highlights the role of renalase gene polymorphism both rs10887800 and rs2576178 (homo and heterozygous) in PE being strongly linked to occurrence of non-severe & severe PE patients. Thus suggesting a promising tool for PE assessment and predicting disease severity.
Keywords
Pre-eclampsia; PCR-RFLP; Renalase gene sequencing
Introduction
Pre-eclampsia (PE) is recognized as a hypertensive multisystem disorder [1]. It is a major cause of maternal and perinatal morbidity and mortality affecting 2% to 8% of all pregnancies [2]. Pre-eclampsia is attributed to a combination of maternal susceptibility with an exaggerated inflammatory response to pregnancy and altered placental function [3]. This lead to release of stress factors that trigger widespread activation of the maternal vascular endothelium [4]. An increase in the level of catecholamines including epinephrine is associated with an increase in blood pressure [5].
Multiple genes such as VEGF&Flt-1and renalase have been studied to identify their role in the pathogenesis of PE. Renalase protein is monoamine oxidase enzyme secreted by the kidneys involved in metabolizing circulating catecholamines [6]. Evidence showed that the increased level of plasma catecholamines markedly augments renalase activity, confirming the contribution of renalase to circulating catecholamine degradation, as well as BP and cardiac function regulation [7].
In this context; the current study designed to evaluate the association between two single‐nucleotide polymorphisms (SNPs), including renalase rs10887800 at and rs2576178 and PE development and severity.
Subjects and Methods
Subjects
This case-control study was carried out at Ain Shams University Hospitals Clinical Pathology Department. The study included (40) Female patients with Pre-eclampsia (group I) recruited from the Out-Patient Clinic and In-Patient Department of Obstetrics and Gynecology at Ain Shams University Hospitals and were further subdivided into group Ia (20) non severe PE, group Ib (20) severe PE based on the disease severity according to ACOG 2017 criteria [8]. In addition, (20) age- and sex- matched healthy subjects served as a healthy control group II. An informed oral consent was obtained from each participant before enrolment in the study. The study was approved by the Research Ethics Committee of Ain Shams University (FMASU MD 118 / 2019).
All subjects with history of PE, twins, diabetes, renal disease, liver dysfunction, chronic hypertension, heart diseases, and hydrops fetal is were excluded from the study. All subjects recruited in the study were subjected to full history taking with emphasis on blood pressure, demographic parameters as maternal age, gestational age, gravidity, parity. Fasting blood glucose (FBG), CBC, BUN, serum creatinine, ALT, AST, urinary dipstick , protein/creatinine ratio and assay of renalase gene rs10887800 and rs2576178 polymorphism by RFLP-PCR were also tested for all studied females.
Sample collection
Under complete aseptic conditions, 7mL of venous blood were withdrawn from all subjects. Samples were divided into 3 vacationers: first 3mL of blood were collected in a sterile plain vacutainer, left to clot for 30 minutes followed by serum separation by centrifugation at 4000 rpm for 10 minutes. Separated serum was used for the immediate assay of FBG, BUN, serum creatinine, AST, ALT. The remaining 4mL of blood were immediately divided into 2 sterile K3 EDTA vacutainer: The first one for immediate assay of CBC and the second tube was stored at -70 °C to be used for subsequent assay rs10887800 and rs2576178. Repeated freezing and thawing were avoided.
Methods
a) Analytical Methods:
Assay of FBG, BUN, serum creatinine, AST, ALT and urinary protein was performed using Beckman Coulter AU 480 Auto-analyzer (Beckman Instruments Inc., Scientific Instruments Division, Fullerton, CA92634, 3100, USA) following manufacturer instructions using reagents supplied by the company.
Complete Blood Count (CBC) was done on Coulter LH 750 hematology analyzer (Coulter Counter T660, Coulter Electronics, Hielaeh, Fl., USA) following manufacturer instructions using reagents supplied by the company.
Urinary protein was tested for by dipstick method (ACON Laboratories, Inc., 10125. Mesa Rim Road, San Diego, CA 92121, USA) following manufacturer instructions.
Calculation of urinary protien/creatinine ratio (reference interval up to 0.2)
Assay of renalase gene rs10887800 and rs2576178 polymorphism by RFLP-PCR:
Steps:
i. Extraction of human genomic DNA was done on EDTA- anticoagulated peripheral whole blood by Thermo Scientific GeneJET Whole Blood Genomic DNA Purification Mini Kit (Thermoscientific, 168 Third Avenue, Waltham, MA, USA) following the manufacturer’s instructions.
ii. Amplification of extracted DNA: Template DNA from previous step was then amplified by using two pairs of oligonucleotide primers (Tab.1. & 2.) for detection of renalase gene polymorphism.
iii. The PCR mixture contained1 μg DNA template, 1 μL forward primer, 1 μL reverse primer,25 μL of Dream Taq Green PCR Master Mix ready to use solution (Thermo scientific, 168 Third Avenue, Waltham, MA, USA.) and nuclease-free water completed to reach a final volume of 50µL.
iv. Amplification was performed on PCR thermal cycler (Thermo scientific, 168 Third Avenue, Waltham, MA, USA.) by denaturation at 95°C for 30 sec, annealing at Tm-5°C for 30 sec, and extension at 72°C for 1 min as many as 35 cycles followed by a final extension at 72°C for 5-10 min.
| Primer 1 (rs10887800 polymorphism) | |
|---|---|
| Forward primer | 5'-CAGGAAAGAAAGAGTTGACAT-3' |
| Reverse primer | 5'-AAGTTGTTCCAGCTACTGT -3' |
| Primer 2 (rs2576178 polymorphism) | |
| Forward primer | 5'-AGCAGAGAAGCAGCTTAACCT-3' |
| Reverse primer | 5'-TATCTGCAAGTCAGCGTAAC -3' |
Tab. 1. Primers used in amplification reaction.
| Parameter | Group I a (non severe) (n=20) n (%) |
Group Ib (severe) (n=20) n (%) |
Group II (control) (n= 20) n (%) |
Test of significance | |||
|---|---|---|---|---|---|---|---|
| Value | p-value | Sig. | |||||
| Gravidity | PG | 7 (35%) | 11 (55%) | 4 (20%) | 0.44(F) | NS | |
| 2 | 7 (35%) | 4 (20%) | 7 (35%) | ||||
| 3 | 5 (25%) | 3 (15%) | 7 (35%) | ||||
| 4 | 1 (5%) | 1 (5%) | 1 (5%) | ||||
| 5 | 0 (0%) | 1 (5%) | 1 (5%) | ||||
| Parity | 0 | 7 (35%) | 11 (55%) | 4 (20%) | 0.44(F) | NS | |
| 1 | 7 (35%) | 4 (20%) | 7 (35%) | ||||
| 2 | 5 (25%) | 3 (15%) | 7 (35%) | ||||
| 3 | 1 (5%) | 1 (5%) | 1 (5%) | ||||
| 4 | 0 (0%) | 1 (5%) | 1 (5%) | ||||
| Maternal age (years) | Mean ± SD | Mean ± SD | Mean ±SD | f= 2.191 | 0.121(A) | NS | |
| 26.2 ± 4.99 | 29.65 ± 6.38 | 29.1±5.34 | |||||
| Gestational age (weeks) | 32.15 ± 2.46 | 31.85 ± 3 | 31.9±2.85 | f= 0.067 | 0.935(A) | NS | |
| Systolic (mmHg) | 144 ± 5.03 | 169.5 ± 10.5 | 113 ± 6.57 | f =268.82 | 0.001(A) | HS | |
| Diastolic (mmHg) | 96 ± 5.03 | 110 ± 6.49 | 73.5 ± 5.87 | f =199.77 | 0.002(A) | HS | |
(A) One Way ANOVA test of significance (f= ANOVA test value).
P>0.05: Non significant (NS), P< 0.05: Significant (S), P< 0.01: Highly significant (S).
Tab. 2. Descriptive Statistics of Different Studied Demographic Data and Pregnancy Data Between Pre-eclamptic Patients Groups and Control Group Using Fisher’s Exact Test and One Way ANOVA test.
RFLP analysis was done using 2 μL of FastDigest green buffer, 10 μL of DNA amplicon, 1 μL of the restriction enzyme and 17 μL of nuclease-free water. PCR products were digested with the appropriate restrictive endonuclease (Msp I for rs2576178 polymorphism and Pst I for rs10887800 polymorphism) at a temperature of 37ºC for 5 min.
Detection of renalase gene rs10887800 and rs2576178 polymorphism: The reaction products were separated by electrophoresis in 2% agarose gel.
Renalase gene rs10887800 and rs2576178 gene sequencing specifications:
Random samples were chosen for gene sequencing on an Automated ABI PRISM 310 Applied BioSystem Genetic Analyzer (Perkin Elmer, Applied Biosystems, Foster city, CA, USA) in order to confirm the exact nucleotide sequence. Steps including conventional PCR were done using the same primers used before and following the previous steps.
PCR products were purified by QIAquick Gel Extraction Kit (QIAGEN, Strasse 1,40724 Hilden, Germany) then sequenced using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems , Thermo-scientific; Singapore) using 4L of Terminator Big Dye, 1L of forward primer, 2L of DNA and 3 uL of DNASE free water where were added, then tubes were placed in the thermal cycler with initial heating step 96°C for 60 seconds then denaturation at 96°C for 10 seconds, annealing at 60°C for 5 seconds, and extension at 60°C for 4 min as many as 25 cycles.
The sequence product was purified using Centri-step Spin Columns Kit then the purified sequence product was subjected to sequencing by capillary electrophoresis.
b) Results Interpretation:
RFLP- PCR:
Restriction digestion for rs10887800 PST 1 polymorphism show (Fig. 1.):
• Wild type AA has single 554 bp band
• Homozygous GG genotype has 2 bands (415, 139 bp)
• Heterozygous AG genotype has 3 bands (554,415 and 115 bp)
Restriction digestion for rs2576178 polymorphism MSP1show:
• Wild type AA has a single 525 bp band
• Homozygous GG genotype has 2 bands (423,102 bp)
• Heterozygous AG genotype has with 3 bands (525, 423 and 102 bp)
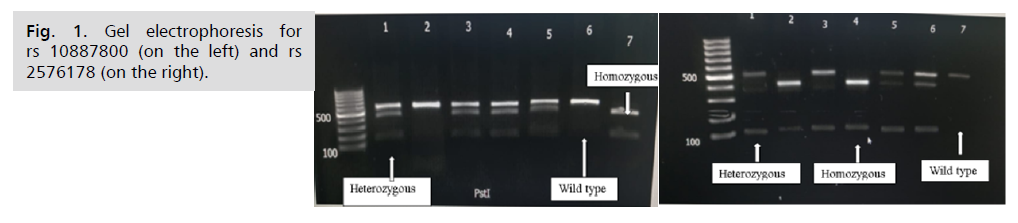
Fig 1. Gel electrophoresis for rs 10887800 (on the left) and rs 2576178. (on the right).
Gene Sequencing (Fig. 2. & 3.)
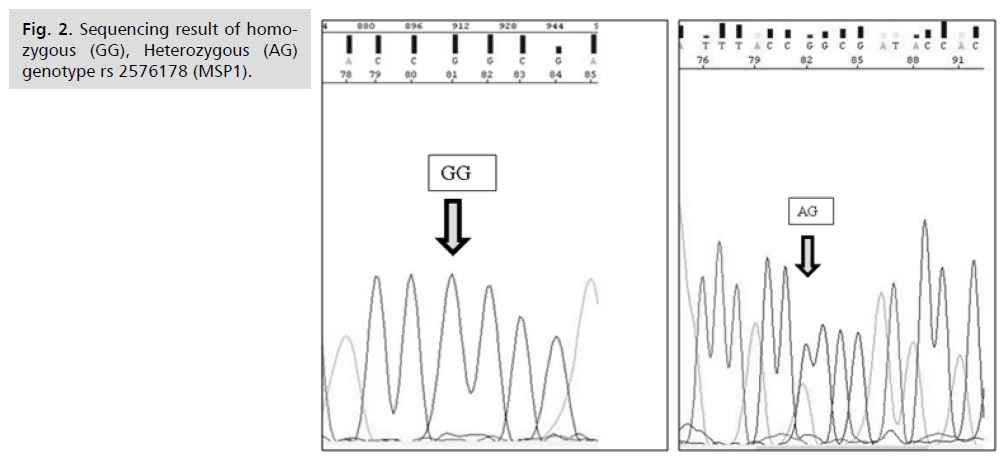
Fig 2. Sequencing result of homozygous (GG), Heterozygous (AG) genotype rs 2576178 (MSP1).
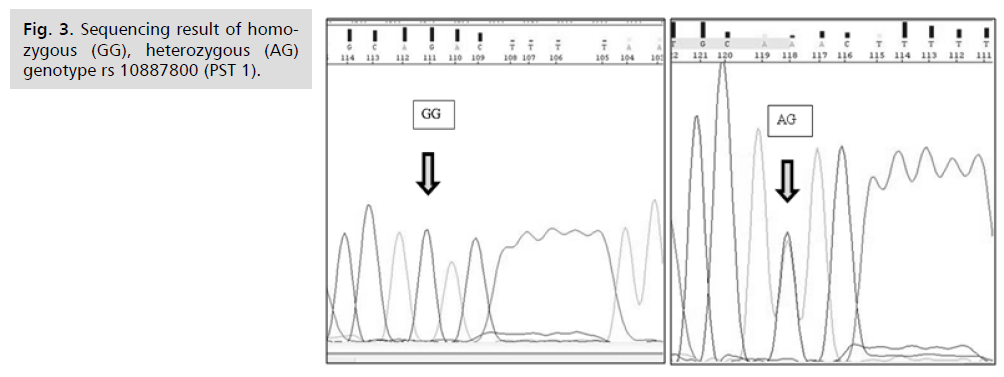
Fig 3. Sequencing result of homozygous (GG), heterozygous (AG) genotype rs 10887800 (PST 1).
Data analysis was done using https://blast.ncbi.nlm.nih.gov/Blast.cgi website.
Statistical Analysis
Data analysis was done using IBM SPSS statistics (V. 22.0, IBM Corp., USA, 2013). Descriptive values were demonstrated as the mean ± SD for parametric data, number and percent were applied to express categorical data. Student t-test was utilized to detect the statistical significance of parametric data between two study group means. Analysis of Variance (ANOVA test) was used to for more than two study group means. Post Hoc Test is used for comparisons of all possible pairs of group means. Chi-Square test was used to examine the relationship between two qualitative variables while, Fisher’s exact test was used to examine the relationship between two qualitative variables when the expected count is less than 5 in more than 20% of cells. p value >0.05 considered statistically non-significant, <0.05 considered statistically significant and <0.01 was considered as highly significant. Odds ratio (OR) with 95% confidence intervals (CIs) was calculated.
Results
Descriptive and comparative statistics of different studied parameters among different studied groups are seen in (Tab. 3. & 4.).
| Parameters | Group I a (n=20) Mean ± SD |
Group I b (n=20) Mean ± SD |
Group II (n=20) Mean ± SD |
One Way ANOVA Test | ||
|---|---|---|---|---|---|---|
| f | P-value | Sig. | ||||
| FBG (mg/dL) | 78.05 ± 4.1 | 78.55 ± 4.26 | 80.8±4.71 | 2.254 | 0.114 | NS |
| Hb (g/dL) | 9.89 ± 1.67 | 8.69 ± 0.95 | 11.43±0.81 | 26.063 | 0.001(A1) | HS |
| WBC (10^3/µL) | 9.19 ± 1.65 | 9.81 ± 1.3 | 7.75 ± 1.26 | 11.123 | 0.002(A2) | HS |
| PLT (10^3/µL) | 202.05 ± 71.88 | 110.8 ± 25.55 | 252.35±51.18 | 36.609 | 0.001(A1) | HS |
| BUN (mg/dL) | 21.15 ± 6.12 | 47.65 ± 22.77 | 16.2 ± 2.28 | 30.588 | 0.001(A3) | HS |
| Creatinine (mg/dL) | 0.92 ± 0.33 | 2.89 ± 1.81 | 0.73 ± 0.13 | 25.271 | 0.001(A3) | HS |
| AST (IU/L) | 19.45 ± 6.24 | 80.05 ± 15.05 | 17.35 ± 2.6 | 279.576 | 0.003(A3) | HS |
| ALT (IU/L) | 22.35 ± 9.29 | 96.3 ± 35.6 | 21.8 ± 6.43 | 78.976 | 0.001(A3) | HS |
| Protien/ Creatinine Ratio | 0.83 ± 0.66 | 2.88 ± 1.11 | 0.13 ± 0.04 | 73.843 | 0.001(A1) | HS |
(A1) Between all groups.
(A2) Control group Vs. (Mild and Severe case groups.)
(A3) Severe cases group Vs. (Control and Mild case groups.)
P>0.05: Non significant (NS), P< 0.05: Significant (S), P< 0.01: Highly significant (S).
Tab. 3. Descriptive and Comparative Statistics Between Group Ia, Group Ib and Group II As Regards Different Studied Parameters Using One Way ANOVA Test.
| Group I (n=40) |
Group II (n=20) |
Chi-square test | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Value | P-value | Sig. | |||
| rs 10887800 (PST 1) |
AA | 13(32.5%) | 16(80%) | χ2= 12.047 | 0.001 | HS | 8.308 (2.31 - 29.88) |
| AG / GG | 27(67.5%) | 4(20%) | |||||
| rs 2576178 (MSP1) |
AA | 11(27.5%) | 17(85%) | χ2= 17.712 | 0.001 | HS | 14.94 (3.65 - 61.19) |
| AG / GG | 29(72.5%) | 3 (15%) | |||||
| Group I (n=40) |
Group II (n=20) |
Fisher’s Exact test | Sig. | ||||
| renelase gene rs 10887800 (PST 1) |
AA | 13(32.5%) | 16(80%) | 0.001(F) | HS | ||
| AG | 19(47.5%) | 4 (20%) | |||||
| GG | 8 (20%) | 0 (0%) | |||||
| rs 2576178 (MSP1) |
AA | 11(27.5%) | 17(85%) | 0.001(F) | HS | ||
| AG | 23(57.5%) | 3 (15%) | |||||
| GG | 6 (15%) | 0 (0%) | |||||
P>0.05: Non significant (NS), P< 0.05: Significant (S), P< 0.01: Highly significant (S).
Tab. 4. Descriptive and Comparative Statistics of Genotype of Renalase (rs 10887800) and (rs 2576178) between Group I and Group II Using Chi-Square Test and Fisher’s Exact Test.
Genotyping of Renalase Gene A>G Polymorphism (rs 10887800) and (rs 2576178) for testing the deviation from Hardy-Weinberg equilibrium was performed by Chi-square test, using the observed genotype frequencies obtained from the data and the expected genotype frequencies obtained from Hardy-Weinberg principle. The distribution of genotypes showed no significant deviation from Hardy-Weinberg Equilibrium among the subjects (χ2 =0.96, p = 0.169) and (χ2 =0, p = 1.0) respectively.
Descriptive and comparative statistics of the genotypes of renalase gene polymorphism (rs 10887800) between pre- eclamptic patients (group I) and healthy controls (group II) showed high statistically significant difference (χ2 = 12.047; p =0.001) as shown in (Tab. 4.). Descriptive and comparative statistics between the control and patient groups regarding allele frequencies of renalase gene A>G polymorphism (rs 10887800) G allele vs A allele increased the disease risk (G vs A , χ2 = 15.271, p = 0.001) .
Descriptive and comparative statistics of the genotypes of renalase gene polymorphism (rs 10887800) between pre- eclamptic patients (group I) and healthy controls (group II) showed high statistically significant difference (Χ2 = 12.047; p =0.001) as shown in (Tab. 4.). Descriptive and comparative statistics between the control and patient groups regarding allele frequencies of renalase gene A>G polymorphism (rs 10887800) G allele vs A allele increased the disease risk (G vs A , Χ2 = 15.271, p = 0.001).
Table 5 illustrates descriptive and comparative statistics of renalase rs 10887800 polymorphism genotype distribution between the two patients subgroups (Ia and Ib) showing non-significant statistical difference between non severe and severe PE (p = 0.212).
| Group I a (n=20) n (%) |
Group I b (n=20) n (%) |
Fisher’s Exact Test | |||
|---|---|---|---|---|---|
| P-value | Sig. | ||||
| renelase gene rs 10887800 (PST 1) | AA | 9 (45%) | 4 (20%) | 0.212 | NS |
| AG | 7 (35%) | 12 (60%) | |||
| GG | 4 (20%) | 4 (20%) | |||
| rs 2576178 (MSP1) | AA | 8 (40%) | 3 (15%) | 0.104 | NS |
| AG | 11 (55%) | 12 (60%) | |||
| GG | 1 (5%) | 5 (25%) | |||
Tab. 5. Descriptive and Comparative Statistics of Renalase (rs 10887800) and (rs 2576178) Genotype Between Group Ia, Group Ib Using Fisher’s Exact Test.
Descriptive and comparative statistics of the genotypes of renalase gene polymorphism (rs2576178) between preeclamptic patients (group I) and healthy controls (group II) showed high statistically significant difference (Χ2 = 17.712; p = 0.001) as shown in (Tab. 4.).
Descriptive and comparative statistics of renalase (rs 2576178) polymorphism genotype distribution between the two patients subgroups (Ia and Ib) show non-significant statistical difference between non severe and severe PE (p = 0.104) (Tab. 5.).
Comparative statistics of different laboratory investigations and blood pressure among group I rs 10887800 (PST1) AA, AG and GG genotypes did not show statistically significant variation in relation to FBG, WBCs, AST and ALT But hemoglobin, platelets, BUN, serum creatinine and protein: creatinine ratio were significantly increased also, SBP and DBP means were illustrated in (Tab. 6.).
| renelase gene rs 10887800 (PST 1) | One Way ANOVA | |||||
|---|---|---|---|---|---|---|
| AA (13) | AG (19) | GG (8) | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | f | p-Value | Sig. | |
| FBG (mg/dL) | 80.31±3.12 | 77.89 ± 4.63 | 76 ± 3.07 | 3.184 | 0.053 | NS |
| Hb (g/dL) | 10.18±1.64 | 9.07 ± 1.3 | 8.35 ± 0.73 | 5.125 | 0.011(A1) | S |
| WBC(10^3/µL) | 8.94±1.05 | 9.81 ± 1.84 | 9.68 ± 1.05 | 1.387 | 0.262 | NS |
| PLT (10^3/µL) | 224.46±67.85 | 130.21 ± 47.02 | 108.13 ± 31.39 | 16.677 | 0.001(A2) | S |
| BUN (mg/dL) | 21.69±6.26 | 37.84 ± 21.65 | 46.88 ± 27.01 | 4.713 | 0.015(A1) | S |
| Creatinine(mg/dL) | 1.02±0.38 | 2.05 ± 1.51 | 2.98 ± 2.39 | 4.427 | 0.019(A1) | S |
| AST (IU/L) | 34.54±26.88 | 56.16 ± 31.17 | 59.25 ± 39.82 | 2.240 | 0.121 | NS |
| ALT (IU/L) | 38.15±29.97 | 63.89 ± 33.39 | 82.88 ± 74.54 | 2.827 | 0.072 | NS |
| Protien/ Creatinine Ratio | 1.04±1.09 | 2.04±1.13 | 2.71 ± 1.73 | 4.827 | 0.014(A1) | S |
| Systolic (mmHg) | 147.69±9.27 | 160.53 ± 13.53 | 162.5 ± 21.21 | 3.965 | 0.028(A1) | S |
| Diastolic (mmHg) | 97.69 ± 7.25 | 105.79 ± 6.92 | 105 ± 13.09 | 3.750 | 0.033(A2) | S |
(A2) AA group Vs. (AG and GG groups).
P>0.05: Non significant (NS), P< 0.05: Significant (S), P< 0.01: Highly significant (S).
Tab. 6. Descriptive and Comparative Statistics of Laboratory Parameters between Renelase Gene alleles of rs 10887800 (PST 1) Among Cases Using One Way ANOVA Test.
Comparative statistics of different laboratory investigations and blood pressure among group I patients with renelase gene rs 2576178 (MSP1) genotypes AA, AG and GG did not show statistically significant variation in relation to FBG, WBCs, ALT and protein: creatinine ratio but hemoglobin, platelets, BUN, serum creatinine and AST were significantly increased. SBP and DBP means were also illustrated in (Tab. 7.).
| Parameters | rs 2576178 (MSP1) | One Way ANOVA | ||||
|---|---|---|---|---|---|---|
| AA (11) | AG (23) | GG (6) | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | f | p-Value | Sig. | |
| FBG (mg/dL) | 79.64 ± 2.91 | 77.48 ± 4.48 | 79 ± 4.52 | 1.122 | 0.336 | NS |
| Hb (g/dL) | 10.05 ± 1.39 | 9.19 ± 1.47 | 8.27 ± 1.03 | 3.282 | 0.049(A1) | S |
| WBC (10^3/µL) | 9.36 ± 1.04 | 9.7 ± 1.81 | 8.95 ± 0.61 | 0.651 | 0.527 | NS |
| PLT (10^3/µL) | 204.55±75.78 | 151.43 ± 60.19 | 87.33 ± 20.07 | 7.271 | 0.002(A1) | S |
| BUN (mg/dL) | 25.82 ± 5.98 | 33.48 ± 22.43 | 53.67 ± 24.9 | 3.894 | 0.029(A1) | S |
| Creatinine(mg/dL) | 1.18 ± 0.41 | 1.78 ± 1.51 | 3.68 ± 2.27 | 5.970 | 0.006(A2) | S |
| AST (IU/L) | 32.36 ± 24.28 | 51.17 ± 32.36 | 76.17 ± 32.5 | 4.088 | 0.025(A1) | S |
| ALT (IU/L) | 36.82 ± 28.15 | 62.78 ± 50.95 | 87.33 ± 31.03 | 2.795 | 0.074 | NS |
| Protien/ Creatinine Ratio | 1.42 ± 1.29 | 1.81 ± 1.41 | 2.8 ± 1.03 | 2.103 | 0.136 | NS |
| Systolic(mmHg) | 150.91±13.75 | 157.39 ± 16.57 | 165 ± 8.37 | 1.771 | 0.184 | NS |
| Diastolic(mmHg) | 100 ± 10 | 103.04 ± 9.26 | 108.33 ± 4.08 | 1.680 | 0.200 | NS |
(A1) AA group Vs. GG group.(A2) GG group Vs. (AA and AG groups.)
P>0.05: Non significant (NS), P< 0.05: Significant (S), P< 0.01: Highly significant (S).
Tab. 7. Descriptive and Comparative Statistics of Laboratory Parameters Between rs 2576178 (MSP1) Gene alleles Among Cases Using One Way ANOVA Test.
Discussion
PE is a serious hypertensive disorder of pregnancy. It corresponds to a specific multisystemic syndrome clinically defined by elevated blood pressure (≥140/90 mmHg) and proteinuria during the second half of the pregnancy [9]. Untreated PE can progress to a severe state identified as eclampsia characterized by edema, hepatic failure, defects in hemostasis, and HELLP syndrome (hemolysis, elevated liver enzymes, and lower platelet levels) [10]. Several risk factors such as immunologic, genetic, environmental, nutritional and infectious origins have been proposed, PE has still remained as a ‘‘disease of theories” [11]. Renalase enzyme plays an important role in regulating sympathetic nervous system activity, hypertension and cardiovascular function through the action of renalase protein on circulating catecholamines degradation and subsequently playing a major role in lowering BP [12]. Various gene polymorphisms can result in structural and functional changes in renalase protein inducing a cascade of vascular damage with unregulated sympathetic nervous system activity. This could be one of the causes that may induce hypertension, vascular complications and various gestational disorders such as PE, pregnancy‐ induced hypertension, and gestational diabetes mellitus [13]. Thus our study aimed to evaluate the association of renalase gene polymorphism (rs10887800 and rs2576178) with the development of PE in pregnant females as well as its degree of severity.
Results of this study show that mutant G allele in rs10887800 was present in 67.5%(27) of female patients with PE. Where homozygous genotype GG represented 20% (8) and heterozygous AG genotype 47% (19) of female patients with PE. Similar results were reported by EL Niadany et al. [14] and li et al. [15] who found a higher distribution of rs10887800 mutant G allele and GG genotypes, the GG genotype represented (40%) & (40%) respectively, in pre-eclamptic patients’ group. On the other hand, studies on Turkish females by Bagci et al. [16] and Iranian females by Teimoori et al. [17] reported (20.9%),(25.7%) for GG genotype of rs10887800 and this may be due to different ethnic distribution.
Meanwhile, in rs10887800, wild type AA genotype represents 80% [16] of our studied control group and interestingly none of them had a mutant homozygous GG genotype which enforces the possible role of G mutant allele in the pathogenesis of PE. Similarly, in the study by EL Niadany et al. [14] and Bagci et al. [16] the wild genotype AA in rs10887800 was found in the majority of control group (58%) and (43%) respectively.
Our study result shows that rs2576178 homozygous and heterozygous genotypes GG / AG were found in 72.5% (29) of group I females with PE. Rs2576178 homozygous GG genotype is seen in 15% (6) while heterozygous AG 57.5% (23) in group I pre-eclamptic patients. On the other hand, wild type AA genotype was found in 85% (17) of control group and interestingly none of the control group had mutant G allele. Our results agreed with a report carried out in Chinese cohort by Li et al. [15] who found a higher distribution of rs2576178 mutant G allele in pre-eclamptic patients where GG genotypes represented (46%) and AG genotype (40%) of cases while the wild genotype AA was seen in (25%) of control group.
In the present study the likelihood of the presence of homozygous and heterozygous genotypes (GG and AG) for rs10887800 in PE patients is expressed by odds ratio (OR) of 8.308 suggesting the development of severe PE with a 95% confidence interval (2.31-29.88). This goes in accordance with the finding of Bagci et al. (16) who reported OR=2.18 with 95%CI = 1.00 – 4.75, EL Niadany et al. [14] (OR=7.25 with 95%CI = 2.81 – 18.83) and li et al. [15] showed OR= 2.42 and 95%CI =1.07 -5.35). In contrast to our results a study by Zhang et al. [12] in Chinese females and de Freitas Moura et al. [10] in Brazilian females, they investigated the potential impact of the rs10887800 polymorphism in the pathogenesis of PE. They reported that the GG genotype was associated with a trend towards a higher risk of PE when compared to controls but without statistical significance (GG vs. AG + AA: OR = 2.16; CI: 0.97–4.86; p = 0.05).
In our study results the odds ratio 14.94 for rs2576178 with 95% confidence interval (3.65-61.19). Similar results were seated by li et al. [15] (OR= 2.49 with 95%CI =1.19 -5.41) for rs2576178 in PE patients.
In our search for the association between the renalase gene polymorphisms (rs10887800 and rs2576178) and the severity of PE a comparison of different genotypes between the two patients subgroups (group Ia m with non-severe PE and group I b with severe PE) was done. The genotype distribution of rs10887800 in group Ia (non severe PE) was present in 4 (20%) for GG homozygous genotype, 7 (35%) for AG heterozygous genotype and 9 (45%) for AA wild genotype. While, group Ib (severe PE) GG was found in 4 (20%) and 12 (60%) in AG and 4 (20%) in AA wild genotype. Comparative statistics showed non-significant statistical difference between non severe and severe PE (p = 0.212 ).
The genotype distribution of rs2576178 polymorphism GG genotype was found in 1 (5%) in group Ia, AG genotype in 11 (55%) and AA genotype in 8 (40%). While, group Ib showed GG genotype in 5 (25%), AG genotype in 12 (60%) and AA genotype in 3 (15%). There were no significant statistical difference for rs2576178 polymorphism genotype distribution between non severe and severe PE (p = 0.104). These result goes in accordance with the study by EL Niadany et al. [14] who found no statistical significance for rs10887800 genotype and Teimoori et al. [17] who found no statistical significance for both (rs10887800 and rs2576178) as regard G allele (in either homozygous or heterozygous) between mild and severe pre-eclamptic patients. Bagci et al. [16] also reported a non-statistical significance for rs2576178 genotype between mild and severe pre-eclamptic patients. However, Bagci et al. [16] study reported that homozygous GG genotype and G allele for rs 10887800 were significantly higher in patients with severe PE when compared to mild PE (p= 0.025, p= 0.022, respectively).
In the present study, Renalase Gene A>G Polymorphism (rs 10887800) and (rs 2576178) was genotyped in all subjects for testing the deviation from Hardy-Weinberg equilibrium. The comparison between observed genotype frequencies obtained from our data and the expected genotype frequencies obtained from Hardy-Weinberg principle showed no significant deviation from Hardy-Weinberg Equilibrium among the subjects (Χ2 =0.96, p >0.05) and (Χ2 =0, p >0.05) respectively. These results go in accordance with li et al. [15] (rs10887800: Χ2 = 0.11, p= 0.73; rs2576178: Χ2 = 0.10, p = 0.75).
In our study, systolic and diastolic BP means were studied among different PE groups in relation to expressed renalase gene genotypes. The high statistical significant difference seen between SBP means among patients with GG/AG genotypes of rs10887800 in females’ patients with PE (n=27) compared to patients with AA (n=13) genotype reflects the highly possible direct relation between the presence of G allele and the elevated SBP encountered in patients with PE. Unfortunately, similar possibility cannot be proven in our study results for rs2576178 renalase gene polymorphism in relation to SBP and DBP means among studied groups.In agreement with the results of our study, Bagci et al. [16] found that the SBP and DBP were significantly higher in females with PE with rs10887800 GG genotype compared with GA or AA genotypes and SBP was significantly higher in females with PE with rs2576178 GG genotype compared with GA or AA genotypes.
These findings reported by our study can be attributed to the action of renalase enzyme on metabolizing catecholamines and maintaining normal SBP and DBP. In animal model a single dose of recombinant renalase (subcutaneously administered 1.3 mg/kg) had an effect equivalent to enalapril (5 mg/kg) and decreased both systolic and diastolic BP Bagci et al. [16]. In the same context, our results linking renalase gene polymorphism rs10887800 to the development of PE and high systolic BP are in accordance with the criteria of diagnosis of PE according to ACOG 2017 [8] and provide a great chance for a possible candidate gene for PE targeted gene therapy.
Our study revealed that the presence of homozygous GG genotype of renelase gene rs 10887800 had a significant statistical difference for hemoglobin, BUN, serum creatinine and protein: creatinine ratio (p= 0.011, 0.015, 0.019, and 0.014, respectively) compared to AA wild genotype. Platelets were significantly higher when both mutant genotypes (GG&AG) were compared to wild genotype AA. Also, for rs 2576178 homozygous GG genotype showed a significant statistical difference for hemoglobin, platelets, BUN, and AST (p=0.049, 0.002, 0.029, 0.025, respectively) compared to AA wild genotype. These results showed the possible role of G allele in the changes accompanied with severe form of PE. Till the time of writing, there were no other study results showing the comparison between mutant genotypes as regard different laboratory parameters.
For enhancement and confirmation of our study results, further sequencing technique on gene product were done using an automated ABI PRISM 310 Applied BioSystem Genetic Analyzer for both suspected polymorphisms rs10887800 and rs2576178. Randomly selected samples from both patients (group Ia, b) and control group underwent sequencing in order to obtain and validate their exact nucleotide sequence. Cases with homozygous mutant genotype (GG) and heterozygous genotype (AG) for rs 10887800 and rs 2576178 by PCR-RFLP confirmed the same result when they were analyzed by sequencing technique which gives our results a robust part of strength for the value of studying renalase gene rs10887800 and rs2576178 polymorphisms in PE.
Conclusion
The present study supports previous results reported by different studies on the association between renalase gene polymorphism rs10887800 and rs2576178 with the development of PE as well as assessment of the disease severity. Also the study highlights the role of renalase gene polymorphism both rs10887800 and rs2576178 (homo and heterozygous genotypes) in PE being strongly linked to occurrence of non-severe & severe PE in patients and the association between the presence of mutant G allele and disease severity assessed by various laboratory and clinical findings. Additionally, our results point out the possible value of using renalase gene as a candidate of targeted gene therapy in PE that need further studies for evaluation.
Recommendations
Further studies with larger sample sizes are recommended to validate our results and to show the effect of renalase polymorphisms in PE pathophysiology as well as assay of serum catecholamines as epinephrine and norepinephrine can be added for further studies for the role of renalase gene in PE.
Authors Contribution
(A) Study Design · (B) Data Collection. (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation (F) Literature Search (G) No Fund Collection
References
- Ives CW, Sinkey R, Rajapreyar I, et al. Preeclampsia—pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(14):1690-702.
- Nirupama R, Divyashree S, Janhavi P, et al. Preeclampsia: Pathophysiology and management. J Gynecol Obstet Hum Reprod. 2021;50(2):101975.
- Aneman I, Pienaar D, Suvakov S, et al. Mechanisms of key innate immune cells in early-and late-onset preeclampsia. Front Immunol. 2020;11:1864.
- Michalczyk M, Celewicz A, Celewicz M, et al. The role of inflammation in the pathogenesis of preeclampsia. Mediators Inflamm. 2020;2020.
- Steinthorsdottir V, McGinnis R, Williams NO, et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun. 2020;11(1):1-4.
- Mochan S, Dhingra MK, Gupta SK, et al. Status of VEGF in preeclampsia and its effect on endoplasmic reticulum stress in placental trophoblast cells. Eur J Obstet Gynecol Reprod: X. 2019;4:100070.
- Czerwińska K, Poręba R, Gać P. Renalase—A new understanding of its enzymatic and non‐enzymatic activity and its implications for future research. Clin Exp Pharmacol Physiol. 2022;49(1):3-9.
- American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, number 222. Obstet Gynecol. 2020;135(6):e237-60.
- Wiles K, Chappell LC, Lightstone L, et al. Updates in Diagnosis and Management of Preeclampsia in Women with CKD. Clin J Am Soc Nephrol. 2020;15(9):1371-80.
- de Freitas Moura MS, Linhares JJ, Noronha EC, et al. Evaluation of the association of the Renalase rs10887800 polymorphism with the risk of preeclampsia in Brazilian women. Pregnancy Hypertens. 2022;27:176-80.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094-112.
- Zhang F, Liu W, Wu Y, et al. Association of renalase gene polymorphisms with the risk of hypertensive disorders of pregnancy in northeastern Han Chinese population. Gynecol Endocrinol. 2020;36(11):986-90.
- Muftin NQ, Al-Garawi ZS, Tahir NT, et al. Renalase: Gene polymorphism and its association with hypertension in some diseases. J Physics: Conf Series. 2021;185: 012054.
- Niadany SS, Abd El Gayed AM, Abd El Gayed EM. Renalase rs10887800 gene polymorphism and its serum level in preeclampsia. Meta Gene. 2020;24:100649.
- Li X, Huang Q, Xu J. Renalase gene polymorphisms and plasma levels are associated with preeclampsia: a hospital-based study in the Chinese cohort. Women & Health. 2021;61(10):957-67.
- Bagci B, Karakus S, Bagci G, et al. Renalase gene polymorphism is associated with increased blood pressure in preeclampsia. Pregnancy Hypertens: Int J Women's Cardiovascular Health. 2016;6(2):115-20.
- Teimoori B, Moradi-Shahrebabak M, Rezaei M, et al. Renalase rs10887800 polymorphism is associated with severe pre‐eclampsia in southeast Iranian women. J Cell Biochem. 2019;120(3):3277-85.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Shimaa Sabry Abd El Ghany1*, Nermine Helmy Mahmoud1, Azza Abd El Rahman Saab1, Mohamed Ahmed Hassan El Kadi2 and Doaa Mostafa Awad12Departments of obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.