Case Report - (2023) Volume 18, Issue 3
Necrotizing fasciitis: A challenging obstetric emergency
Sahadete Shala, Merita Krasniqi, Brikene Elshani, Astrit M.Gashi*, Jehona Luta, Besa Selimi and Gent HaxhikadrijaReceived: 01-Sep-2023, Manuscript No. gpmp-23-121936; Editor assigned: 04-Sep-2023, Pre QC No. P-121936; Reviewed: 12-Sep-2023, QC No. Q-121936; Revised: 19-Sep-2023, Manuscript No. R-121936; Published: 29-Sep-2023
Abstract
Background: Necrotizing fasciitis (NF) is a rare but potentially life-threatening infection that primarily affects subcutaneous fascia, with rapid spread to surrounding tissues. In this retrospective case study, we investigated the clinical presentation, management, and outcomes of NF in a 28-year-old primipara with multiple risk factors, including type I obesity, poorly controlled diabetes, hypothyroidism, a history of recurrent abortions, and an allergic reaction to Cephalosporins, who developed NF following a scheduled cesarean delivery.
Methods: Detailed medical records were retrieved, including pre-operative, perioperative, and postoperative data. Microbiological data from wound cultures and antibiotic sensitivity patterns were analyzed.
Results: The patient presented with erythema and tenderness above the suprapubic operative wound on the 7th postoperative day. Urgent necrectomy, radical debridement, and hemostasis were performed, with positive wound cultures for Streptococcus agalactia gr. B. Subsequent microbiological analysis revealed Acinetobacter baumannii, sensitive only to Colistin. Treatment with Colistin led to successful eradication of the infection and formation of granulation tissue. The patient was discharged from the hospital in good physical condition after appropriate counseling and therapy.
Conclusions: This case study sheds light on the clinical course and management of NF in high-risk obstetric patients following cesarean delivery. Prompt recognition, early surgical intervention, and targeted antibiotic therapy were crucial for successful treatment. Further research is needed to improve early detection and treatment strategies for NF in obstetric patients with multiple risk factors. The findings emphasize the importance of vigilance in identifying NF in postoperative patients and implementing timely and appropriate interventions to achieve positive outcomes.
Keywords
Necrotizing fasciitis; Cesarean delivery; Surgical intervention; Postoperative infection; Kosovo
Introduction
Necrotizing fasciitis NF has first been described by Hippocrates, to which was referred to as the flesh-eating disease. While the term NF was first presented by Wilson in 1953, its first association with cesarean delivery was reported by Gretz et al., [1-3]. Necrotizing fasciitis is an acute infection, with rapidly evolving events. In 80% of cases a breach in skin’s integrity is followed by bacterial infection [4]. Leading to inflammation and progressive necrosis from dermis, to adjacent muscles and skin [4-8]. Most patients at the infected site present with erythema, swelling, and pain.4 NF after Cesarean delivery is rare, but its progression has been described worse than in non-obstetric population [9,10]. NF is characterized with vague and nonspecific initial signs that can delay and mask the diagnosis [4,8-11]. NF in postoperative patients represents only 20% of all cases [12].
Risk factors associated with NF after C- section are diabetes, obesity, alcoholism, hypertension, non-steroidal anti-inflammatory drugs (NSAID) use, peripheral vascular disease, childbirth, anemia and socioeconomic background [2,4,6,8-10,13]. Even though there are cases of NF without risk factors, patients presented with them should be categorized as high-risk patients [10]. Surgery is an independent risk factor [5]. Goh et al systematic review presented 9 studies that included 1463 patients, 8 studies out of 9 described diabetes as the most common comorbidity [12].
Based on causative agent, NF after cesarean delivery is mostly polymicrobial also known as type I, is associated with comorbidities like Diabetes, cultures are positive for Gram positive organisms such as Staphylococcus aureus, Streptococcus pyogenes, and enterococci, Gram negatives such as Escherichia Coli and Pseudomonas species, and anaerobes like Bacteroides and Clostridium species. While monomicrobia which are encountered less often are also NF known as type II, causative agents are mostly skin derived agents such as Gram-positive cocci [2,4,6,12,14]. Up to one third of patient’s methicillin resistant Staphylococcus aureus (MRSA) has been isolated [5]. For type III NF are responsible marine related organisms, and much rarely type IV NF is encountered, caused by fungal infection [15].
Pivotal to survival of these patients is prompt diagnosis and immediate surgical treatment [3]. Considering that only 15-34% of cases have a correct diagnosis at initial presentation [6].
Since most of the patients when developing NF are already covered with antibiotics, the most important treatment step is surgical management by serial debridement and surgical excision of necrotic tissue.
Outcomes are almost always fatal when conservative therapy is applied without surgical intervention [3,9].
Case Report
A 28 year’s old primipara, at 37 +5 weeks of gestation underwent a scheduled cesarean delivery under spinal anesthesia because of her remarkable medical history. The patient had type I obesity BMI 34,4kg/m2 poorly controlled diabetes diagnosed 4 years ago, hypothyroidism diagnosed 3 years ago, a history of recurrent abortions and a positive history of allergic reaction to Cephalosporins. She gave birth to a live male baby W:3730gr, L:53cm, HC:36cm and Apgar Score 7/8.
After 24h in the intensive care unit, the patient was transferred to the ward, in a good condition where she continued treatment with 1.5 g of Metronidazole I.V. solution and 1.5g Erythromycin tablets, subcutaneous anticoagulants, levothyroxine and oral antidiabetics. Because of a nonspecific abdominal pain IM Diclofenac sodium solution was administered. Her vital signs on the first day in the ward were within normal range: respiratory rate 15/min, body temperature 36.7 °C, heart rate 81/min and blood pressure 110/70mmHg. White cell count and C reactive protein were also in the normal range. Everyday wound inspection, care with wet and dry dressing were performed. On the 7th postoperative day, while still in therapy during physical examination a 4 cm tender erythema above the supra pubic operative wound was revealed. Just hours later an area of in duration above the umbilical region, and an ecchymotic lesion about 5.0 x 5.0 cm, with a central area of excoriation with yellow crust was noted.
Necrotizing fasciitis is immediately suspected and a plastic Surgeon is consulted. First wound culture came positive for Streptococcus agalactia gr. B. WBC: 33.8X103/mm3; Glucose: 12.16mmol/l, Urea 3.94 mmol/l, Creatinine 61.3 umol/l; CRP 267.8 mg/l; and Procalcitonin 0.24 ng/ml. After consulting with Infectologist, based on antibiogram, 1.5 g Vancomycin and 1.5g Imipenem are started. After 24 hours during examination, anincrease in the area of induration approximately 10x11 cm is observed, with a central necrotic area, sightly grayish in the periphery within the ecchymotic- purple lesion. Urgent necrectomy, radical debridement, hemostasis is performed under spinal anesthesia. Necrotic subcutaneous fascia and the anterior portion ofrectal sheath are removed, with sparing of rectal muscles. Labs after necrectomy and radical debridement: WBC 20.8X 103/mm3, CRP 169.8 mg/l; glucose 6.38mmol/l; procalcitonin 0.27 ng/ml.
After 48h of necrectomy and radical debridement, the microbiological swab from the wound is taken again. The culture result came positive for Acineto bacter baumannii, a nosocomial bacterium sensitive only to Colistin. Treatment with Colistin continues 3 000 000 IU three times per day for 7 days. After 7 days of treatment, the microbiological swab is repeated, and no bacteria were found in the wound. The formation of granulation tissue is observed, and the patient’s general condition was very good. Vital signs after 12 days of necrectomy: respiratory rate 14/min, temperature 36.4 °C, pulse 80/min, blood pressure 120/80mmHg. On 12th day excision and curettage of the granulomatous tissue is performed as well abdominal reconstruction and a subcutaneous drainage was placed. The wound is cleaned every day and the patient discharged from the hospital in very good physical condition with counseling and therapy.
Fig. 1. (a) shows the second day of visible skin changes. Ecchymotic periphery with grayish/ darker center, a bulla in the center has erupted. Fig. 1. (b) shows the induration and erythema slightly over umbilical region. 3rd - preoperative picture. Notice darker periphery and necrotic center, with a crust in the center. Fig. 1. (c) 4th day after radical debridement and surgical removal of necrotic tissue.
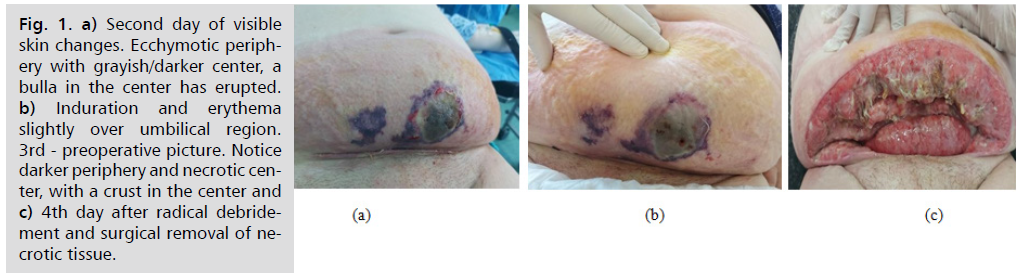
Fig. 1. a) Second day of visible skin changes. Ecchymotic periphery with grayish/darker center, a bulla in the center has erupted. b) Induration and erythema slightly over umbilical region. 3rd - preoperative picture. Notice darker periphery and necrotic center, with a crust in the center and c) 4th day after radical debridement and surgical removal of necrotic tissue.
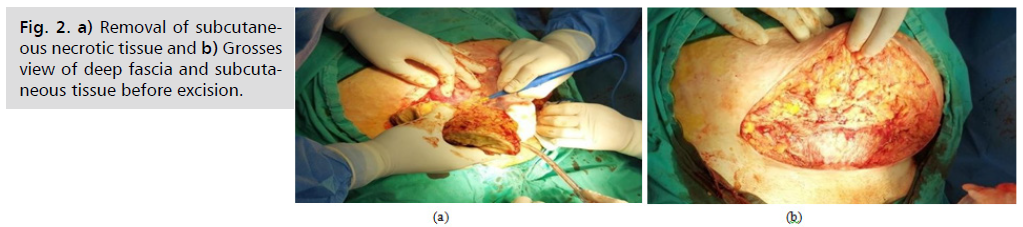
Fig. 2. (a) Shows the removal of subcutaneous necrotic tissue; Fig. 2. (b) shows the grosses view of deep fascia and subcutaneous tissue before excision.
Fig. 2. a) Removal of subcutaneous necrotic tissue and b) Grosses view of deep fascia and subcutaneous tissue before excision.
Discussion
NF is a rare progressive infection that primary affects subcutaneous fascia, but rapidly may spread to the rectal sheath and skin. Known for her vigorous spread to the surrounding tissues, with initial unspecific symptoms, prompt surgical intervention of the necrotic tissue is recommended to prevent fulminant deterioration to septic shock, multi-organ failure and death respectively [1-3,16,17].
Mortality rate from NF can reach 76% [2,9].
An increase in incidence was reported by Out et al between 2001 - 2010, partially because of over diagnosis, by generalizing all soft tissue infections as NF; from the increased incidence of co morbidities like diabetes, obesity, and partially because of the altered maternal immune system [8].
Goepfert retrospective analysis of 8 years revealed from 5048 cesarean deliveries 9 cases complicated by NF. Presenting an incidence rate of 1.8 per 1000 [3].
The most common location of the NF is limbs, perineum and abdomen [1,2,6]. Abdominal NF also known as Meleney’s gangrene, while Fournier’s gangrene encompasses perineal and genitals necrotizing fasciitis [15].
Locally NF, initially presents with erythema and warmth, but rapid increase in tenderness and edema exceeds the erythematous borders. Edema produces tense skin, referred as “wooden” during palpation. Progression of infection manifests with skin vesicles, bullae, ecchymosis, paresthesia/anesthesia. Subcutaneous emphysema and crepitus are present in the gas-producing type of NF, while their absence does not rule out the disease [4,18]. Pathognomonic of NF is the discrepancy between local manifestations and pain severity, with the latter being significantly severe; this is also known as a warning sign. In some cases, in absence of local signs, systemic septic shock signs develop progressively. This should always be proceeded with empiric therapy [1,2,4,10]. Agonizing pain in cases of NF was first described by Meleney [9,11,14]. In some cases of NF hypocalcemia is noted from bacterial lipases action over subcutaneous fat, and hyperbilirubinemia from bacterial hemolysins [11]. Lymphangitis is rarely associated with NF, usually from the primary insult affecting fascia.4 NF in early stages infection tends to spread in a horizontal plane (fascial spread), then later stages, vertical spread happens by affecting the rectal sheath, muscles and skin [15]. Because of the nonspecific signs and symptoms the initial phase of NF is mistaken for cellulitis or wound hematoma [10]. Also Clinicians must be able to quickly distinct cellulitis, which can be managed with antimicrobials, from NF that requires surgical intervention, where patients experience severe pain, and can propagate easily to tissue necrosis and hemodynamical instability [16]. Histopathologic sections of superficial/ deep fascial necrosis have shown small/ medium sized vessels occluded by thrombi and necrotic changes in eccrine glands and ducts too. Treatment delay or inadequate dosage/ medication of choice could lead to extension of necrotic process to the muscles, resulting in myositis or myonecrosis. The correlation between histopathologic findings of debrided tissue in patients with NF and the clinical outcome was adopted as a prognostic tool by Bak-leh et al. [4].
As subcutaneous tissue vessels get obstructed by thrombi, and superficial nerves fibers get destroyed, pain in reduced manifesting with anesthesia [18].
Bacterial overgrowth is supported by compromise in skin or mucosal integrity, hyperglycemia and systemic immunosuppression. Tissue necrosis and spread of causative agents, is fascilitated by their enzymes and toxins. Progression of tissue necrosis is as fast as 1 inch per hour. Impaired blood flow to the fascial layers beneath skin, makes them prone of hypoxic events, respectively necrosis [19].
In a retrospective study on patients with NF cutaneous signs changes were recorded while the disease evolved, from day 0 to day 4. At initial exam patients had erythema, warmth, tenderness, induration, edema, and as late signs with elapsed time (day 4) patients developed blisters, anesthesia, necrosis and skin crepitus, multi-organ failure [1,4].
Our case is characterized by monomicrobial NF. In two separate occasions were isolated two different causative agents. At Barant et al vaginal swab and hemoculture isolated Group B Streptococcus Agalactiae.
This is thought to be favored by the emergency condition of cesarean delivery, with dilated cervix and ruptured membranes [8]. In our case a positive wound culture for Group B Streptococcus agalactiae resulted in circumstances of a scheduled C-section, closed cervix and intact membranes.
Post-operative NSAID has been reported as a risk factor. In our case, the patient was administered NSAID in the immediate postoperative period, besides that, this patient had a few other preoperative risk factors. The mechanism through which NSAID favor progression of NF is thought to be delay of diagnosis by masking signs and symptoms, by inhibiting chemotaxis, phagocytosis or by decreasing lymphocyte response [2,5,6,19]. Causative agents associated with fatal outcome have been described by Alvi et al, and Gallup et al, where rare agents such as zygomycosis, C. perfringes and C. sordellii were isolated [9,13,20].
Facilitating the diagnosis of NF are imaging modalities. Ultrasound can determine the presence of edema, abscess or emphysema along the fascial plane. While CT has a specificity of 80–98 % in detection of NF, especially distinguishing between cellulitis and NF, in early stages has little contribution [2,4,6]. Plainradiologic films have been shown to detect gas in Camper’s fascia and muscles only in 35% of cases. Needle aspiration and incisional biopsy have a limited use because of false negative results [1]. Gold standard modality is biopsy during or after debridement, where direct visualization of fascial planes and muscles is achieved [1,3].
Also finger test has been described to help in diagnosing NF; under local anesthesia a 2cm incision of affected area is made. The test is positive when index finger will dissect easily subcutaneous fascia from deep one, also fascial resistance, foul smell fluid and active bleeding will be absent [12,15]. Wong et al developed the LRINEC scoring test, constituted by certain laboratory values such as: C reactive protein, hemoglobin, total leucocyte count, sodium, creatinine and glucose serum levels. Points more than 8, have a positive prediction for progression of infection towards NF [10,15].
An important step during surgical treatment is serial debridement’s, which may also limit the size of the area that needs reconstruction. Also delaying abdominal closure is associated with prevention of compartment syndrome [3,6]. A North Carolina study revealed the impact of immediate surgical intervention. 11 from 15 women had a fatal outcome then the surgery was delayed > 48hours [14].
IV antibiotics should be started immediately and later changed based on sensitivity [1]. Crystalloids, colloides, sometimes vasoactive agents, even inotropic agents must be prescribed [16].
Surgical management consists on removing all non-viable fascias, with a mandatory surgical exploration after 48 hours to ensure progression of infection has stopped. Repeated depridements must be performed. Reconstruction should be considered only after fully eradicated infection [1].
Management of NF with I.V. immunoglobulin and hyperbaric oxygen remains contraversal [2,16].
Most patients with NF take long courses of treatment. Carlson Babila Sama et al presented a case of a NF, where after extensive debridement of necrotic tissues, abdominal wound was left open to heal by secondary intention. A complete closure of abdominal defect was achieved, by week 15 and a complete wound closure by week 25 respectively [3].
Conclusion
Necrotizing fasciitis must always be considered in an obstetric patient with an escalating pain that doesn’t correspond to physical signs. Patients with risk factors such as diabetes, obesity and severe anemia, should be considered a high-risk group. Immediate surgical intervention with repeated debridement, lowers the rate of complications, increases good outcomes for the patient.
References
- Hasham S, Matteucci P, Stanley PR, et al. Necrotising fasciitis. BMJ. 2005;330(7495):830-833.
- Castro AG, Rodriguez-Borregan JC, Obeso T, et al. Necrotizing fasciitis after cesarean section. Arch Gynecol Obstet. 2008;277:579-81.
- Sama CB, Tankou CS, Angwafo III FF. Fulminating postcaesarean necrotising fasciitis: a rare and lethal condition successfully managed in a resource-disadvantaged setting in sub-Saharan Africa. Case Rep Obstet Gynecol. 2017;2017.
- Salati SA. Necrotizing fasciitis–a review. Pol J Surg. 2022;95(2):1-8.
- Kumari A, Kundan M. A Rare Presentation of Fulminant Necrotizing Soft Tissue Infection in Post-Partum Period: Report of Two Cases. Int J Infect. 2020;7(1).
- DeMuro JP, Hanna AF, Chalas E, et al. Polymicrobial abdominal wall necrotizing fasciitis after cesarean section. J Surg Case Rep. 2012;2012(9):10.
- Damisa J, Ahmed S, Harrison S. Necrotising fasciitis: a narrative review of the literature. Br J Hosp Med. 2021;82(4):1-9.
- Barant S, Radbata D, Oberweis D, et al. Fasciite nécrosante de la paroi abdominale post-césarienne. Rev Med Brux. 2016;37:178-82.
- Gallup DG, Freedman MA, Meguiar RV, et al. Necrotizing fasciitis in gynecologic and obstetric patients: a surgical emergency. Am J Obstet Gynecol. 2002;187(2):305-11.
- Medhi R, Rai S, Das A, et al. Necrotizing fasciitis–a rare complication following common obstetric operative procedures: report of two cases. Int J Womens Health. 2015:357-60.
- Pauzner D, Wolman I, Abramov L, et al. Post-cesarean-section necrotizing fasciitis: report of a case and review of the literature. Gynecol Obstet Invest. 1994;37(1):59-62.
- Goh T, Goh LG, Ang CH, et al. Early diagnosis of necrotizing fasciitis. J Br Surg. 2014;101(1):e119-125.
- Rimawi BH, Graybill W, Pierce JY, et al. Necrotizing fasciitis and toxic shock syndrome from clostridium septicum following a term cesarean delivery. Case Rep Obstet Gynecol. 2014;2014.
- Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther. 2005;3(2):279-94.
- Shaikh N, Khawaiter J, Al-Thani H. Necrotizing fasciitis: a surgical and medical emergency. Surg Sci. 2012; 3(11):518-525.
- Chen LL, Fasolka B, Treacy C. Necrotizing fasciitis: A comprehensive review. Nursing. 2020;50(9):34.
- Kapp DL, Rogers M, Hermans MH. Necrotizing fasciitis: an overview and 2 illustrative cases. Int J Low Extrem Wounds. 2018;17(4):295-300.
- Wallace HA, Perera TB. Necrotizing Fasciitis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Ahn C. Necrotizing fasciitis: reviewing the causes and treatment strategies. Adv Wound Care. 2007;20(5):288-93.
- Alvi AR, Shamsi G. Rhizopus necrotizing fasciitis of caesarean wound: A rare life threatening condition. J Coll Physicians Surg Pak. 19(9),579-581.
Author Info
Sahadete Shala, Merita Krasniqi, Brikene Elshani, Astrit M.Gashi*, Jehona Luta, Besa Selimi and Gent HaxhikadrijaCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.