Research - (2024) Volume 19, Issue 4
Maternal safety of pyridoxine and doxylamine combined for pregnancy-related nausea and vomiting
Widad Abd AL-Jabbar1*, Nada Abdulhur Abed Alibraheemi2, Yasmeen Ali Hussein2,3, Saad Mashkoor Waleed4 and Najah R. Hadi5Received: 01-Nov-2024, Manuscript No. gpmp-24-151566; Editor assigned: 04-Nov-2024, Pre QC No. P-151566; Reviewed: 18-Nov-2024, QC No. Q-151566; Revised: 30-Nov-2024, Manuscript No. R-151566; Published: 30-Dec-2024
Abstract
Background: Up to ninety percent of pregnant mothers experience nausea and vomiting in pregnancy (NVP) which is the most prevalent medical condition during pregnancy. Hyperemesis gravidarum (HG), a severe prognosis form of nausea and vomiting, affects 0.3% to 3.6% of pregnant mothers in the world. HG is lead to fetal and maternal morbidity as well as nutritional deficiencies during pregnancy period in addition to its effect on mentality, injury to oesophagous, and birth abnormalities such as premature delivery, low birth weight. The purpose of the present research is to assessing the safety of doxylamine & pyridoxine combination in nausea and vomiting in pregnancy compared to B6 only.
Patients and methods: One hundred fifty pregnant women suffering from nausea and vomiting during pregnancy were enrolled in this study. Randomized divided into two groups, group 1 (n= 75) receive (doxylamine and B6) and group 2 (n= 75) receive B6 only for 15 days with a dosages varying from 2–4 tablets \ day, Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) score were assessed at 0 time and at three, six, fifteen day of study, blood samples were collected at 0, and 15 day of study for determination of Ferritin & potassium level.
Results: Doxylamine 10 mg and pyridoxine 10mg (B6) use has a good effect in controlling N&V in pregnancy over B6 only.
Conclusions: Doxylamine–pyridoxine together is well absorbed in pregnancy and very safe specially when taken as a prescribed dose of up to four tablets per day for treating nausea and vomiting during pregnancy.
Keywords
Nausea; Vomiting; Pregnancy; Hyperemesis gravidarum
Abstract
Background: Up to ninety percent of pregnant mothers experience nausea and vomiting in pregnancy (NVP) which is the most prevalent medical condition during pregnancy. Hyperemesis gravidarum (HG), a severe prognosis form of nausea and vomiting, affects 0.3% to 3.6% of pregnant mothers in the world. HG is lead to fetal and maternal morbidity as well as nutritional deficiencies during pregnancy period in addition to its effect on mentality, injury to oesophagous, and birth abnormalities such as premature delivery, low birth weight. The purpose of the present research is to assessing the safety of doxylamine & pyridoxine combination in nausea and vomiting in pregnancy compared to B6 only.
Patients and methods: One hundred fifty pregnant women suffering from nausea and vomiting during pregnancy were enrolled in this study. Randomized divided into two groups, group 1 (n= 75) receive (doxylamine and B6) and group 2 (n= 75) receive B6 only for 15 days with a dosages varying from 2–4 tablets \ day, Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) score were assessed at 0 time and at three, six, fifteen day of study, blood samples were collected at 0, and 15 day of study for determination of Ferritin & potassium level.
Results: Doxylamine 10 mg and pyridoxine 10mg (B6) use has a good effect in controlling N&V in pregnancy over B6 only.
Conclusions: Doxylamine–pyridoxine together is well absorbed in pregnancy and very safe specially when taken as a prescribed dose of up to four tablets per day for treating nausea and vomiting during pregnancy.
Introduction
Pregnancy-associated nausea and vomiting (NVP) is common and can impact about 50% to 90% of pregnancies, it is connected with a higher risk of recurrence in following pregnancies, symptoms are moderate and limited to the first trimester, ending by 20 weeks of gestation; Nevertheless, up to 2% of pregnancies may encounter hyperemesis [1]. Those with even moderate to mild symptoms may report important psychosocial discomfort from disturbance of daily activities, unable to complete social and proficient accountability, or stress on relationships, in addition to the potential for intrauterine growth retardation (IUGR) or premature delivery as a repercussion of severe symptoms [2]. Ability to distinguish NVP from other causes of these symptoms that would need substitute treatments is equally significant, given the possible psychological and medical effects of NVP on the fetus and mother, treatment and appreciation of NVP are important even if mild to moderate symptoms, Up to 20% of women experiencing NVP may use pharmaceutical treatment [3].Treatment includes vitamin supplementation, anti-emetic drugs in addition to Thrombo-prophylaxis, make correcting for hypovolemia, ketosis and electrolyte imbalances, severity of the case assessed by modified twenty four hr. PUQE ( Pregnancy Unique Quantification of Emesis & Nausea) score it is a verified evaluation method which take into a count of health status when assessing the seriousness of nausea vomiting in pregnancy (NVP), It is a useful tool for choosing the treatment plan [4].The Modified 24-hour PUQE Score is assessed as:
1. Typically within a day, how long has she been experiencing nausea or feeling sick to her stomach? (Tab. 1.)
| Manifestation | Nothing at all | >1 hour | 2-3 hours | 4-6 hours | > 6 hours |
|---|---|---|---|---|---|
| Score | 1 | 2 | 3 | 4 | 5 |
Tab. 1. Experiencing nausea or feeling sick to her stomach score in a day.
2. Typically within a day, has she thrown up \or vomited? (Tab. 2.)
| Manifestation | < 7 times | 5-6 times | 3-4 times | 1-2 times | didn't throw up |
|---|---|---|---|---|---|
| Score | 5 | 4 | 3 | 2 | 1 |
Tab. 2. Experience of thrown up \or vomited in a day.
3. Typically within a day, how often did she retching or have dry heaves without how many times she had retching or dry heaves without delivering anything? (Tab. 3.)
| Manifestation | < 7 times | 5-6 times | 3-4 times | 1-2 times | didn't throw up |
|---|---|---|---|---|---|
| Score | 5 | 4 | 3 | 2 | 1 |
Tab. 3. Experience of retching or have dry heaves in a day.
So, the key is: mild ≤ 6, moderate 7 – 12 and the severe ≥ 13.
If the pregnant’s PUQE score is 7–12, treatment choices include antiemetic medications to ease her anxiety and permit her to rest, dietary intake of frequent, small servings of her favorite foods and oral fluid encouragement, In order to exclude a molar or multiple pregnancy (which is more likely to result in nausea and vomiting), establish just one healthy pregnancy and arrange for ultrasound, Weigh the pregnant woman and note her weight in the management and assessment checklist, The woman should be demanded not to self-medicate and to continue taking the antiemetic as directed [5]. Perinatal vitamins with iron can prevent and treat iron deficiency anemia with pregnancy, 27 milligrams of iron per day is the requirement of pregnant woman, Nausea and vomiting are thought to be risk indicators for iron deficiency anemia [6]. Blood protein ferritin is consist of iron, test of ferritin could be used to determine the body store of iron ; if the outcome indicates a low level of blood ferritin , it is thought that the body is iron deficient , which may result in anemia [7]. Hypokalemia is among the most prevalent disorders of fluid and electrolyte imbalance in medical practice, 3.5 to 5.5 mEq/L is the normal potassium level in blood; if the level below 3.5 mEq\L hypokalemia is considered, In severe cases, hypokalemia may be lead to muscle weakness and cardiac arrhythmia, cause of low serum potassium level in hypokalemic patients with a low total body potassium store include excessive potassium loss from the skin, gastrointestinal system, or kidneys, inadequate potassium consumption, and potassium consumption as a result of increased cell production [8]. Because nausea and vomiting had a significant effect in lives to woman in pregnancy, it is imperative that it be treated well, In addition, it could be more difficult to manage symptoms if treatment is delayed [9]. As soon as possible it is advised to treat NVP symptoms to delay them from getting worse and to prevent complications like hospitalization, The Food and Drug Administration (FDA) approved many types of drugs for the treatment of NVP in cases where conservative treatment is ineffective [10].
Patients and Methods
The study was performed at Hilla City's Maternity and Pediatrics Teaching Hospital. Over all 150 pregnant women with NVP who were eighteen years of old or older and in the 7–14 week gestational age range were included in this study. After the physical examination, tests in laboratories (total ferritin and potassium in blood level), and ultrasonography verification in utero to make sure of singleton pregnancies, women were randomly assigned to two groups: group 1 (n=75) received both doxylamine and pyridoxine 10 mg. Group 2 (n=75) received vitamin B6 (pyridoxine 10 mg) exclusively for 15 Days with a dose varying from (2–4) tablets per one day had a PUQE score ≥ 6) [11]. The antiemetic drug was given at night on first day. If nausea & vomiting symptoms continued throughout the afternoon hours of second day (i.e., PUQE Score above 6), On Day 3, the patients was advised to take her normal dosage of two tablets before bed and one more tablet the next morning Considering the patients' evaluation, to control symptoms that arise during night, the patient may have been advised to take another tablet in the mid-afternoon of the day, the lowest prescribed study medicine was two tablets taken at bedtime, with the maximum dosage of four tablets per day as needed depending on the patient's symptoms included timing, duration, intensity, and frequency [12]. There were 14 dosing days during the study's 15-day duration. On Days 3 (+/− 1 day), 6 (+/− 1 day), and 15 (+/− 1 day; end of study visit), pregnant woman went back to hospital in the morning before her morning dose to pick up their daily notes & finish any information related study. On Days 3, 6, and 15, patients simultaneously completed the Pregnancy-Unique Quantification of Emesis (PUQE) score.
Statistical Analysis
All data were examined using the SPSS software version, which involved repeated measurements and the calculation of the least significant difference (LSD) (22). P values greater than 0.05 were thought to indicate statistical non-significant, if values of P< 0.05 that’s mean statistical significance.
Results
The percentage of PUQE for the (Doxylamine and B6) group was significantly lower (p<0.05) than for the B6 group, and the blood levels of potassium and ferritin for the Doxylamine and B6 groups were significantly higher (p<0.05) than those of the B6 group. Fig. 1. 2. and 3. show the outcomes.
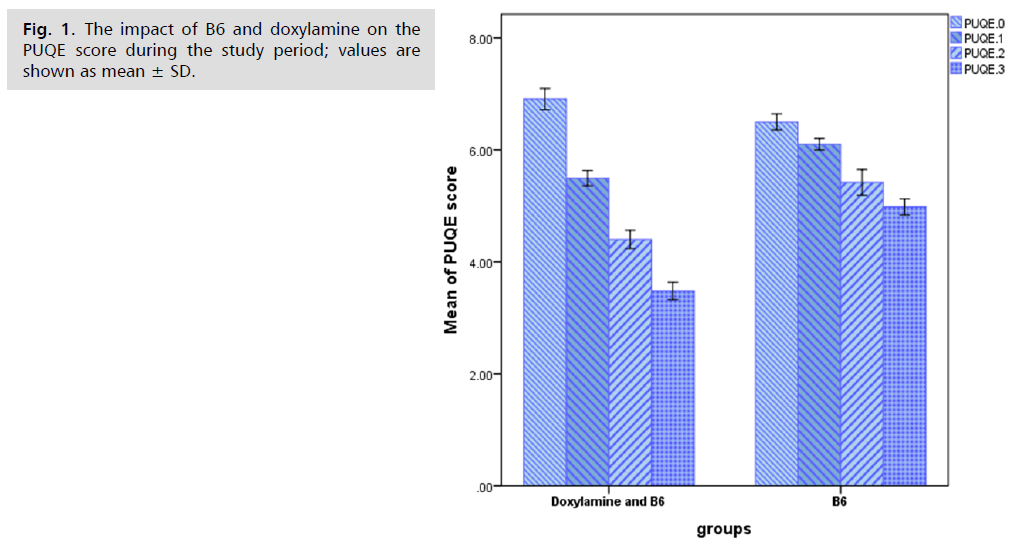
Fig. 1. The impact of B6 and doxylamine on the PUQE score during the study period; values are shown as mean ± SD.
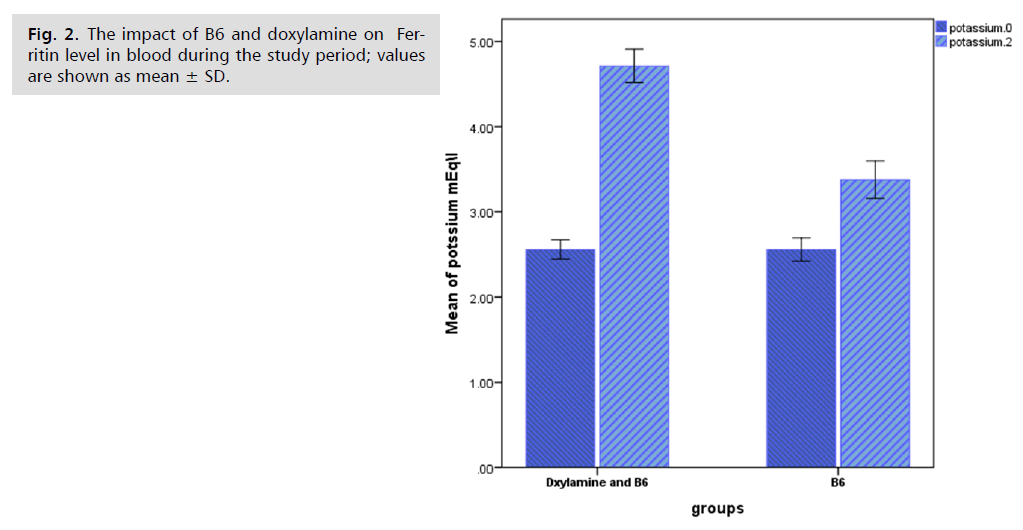
Fig. 2. The impact of B6 and doxylamine on Ferritin level in blood during the study period; values are shown as mean ± SD.
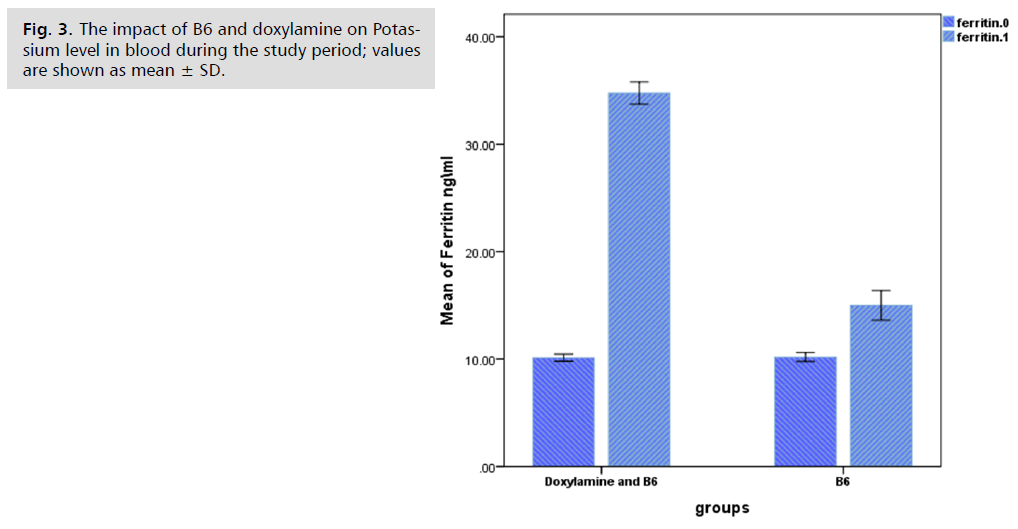
Fig. 3. The impact of B6 and doxylamine on Potassium level in blood during the study period; values are shown as mean ± SD.
Discussion
It is suggested to control NVP symptoms as soon as possible to stop them from getting worse and to stop complications including hospital stays [13]. Alteration in diet and lifestyle can successfully decrease symptoms in women with mild NVP [14]. Women with moderate to severe NVP symptoms should call with their doctor and obseve into pharmacologic therapy if their symptoms don't improve [15]. Numerous of H1 receptor antagonists, including doxylamine, have proven as an efficient and secure for the treatment of NVP [16]. The medications used in NVP in current study work by interfering with the vestibular nausea route, which ultimately affects the transduction of signal in the vomiting center. Additionally, many studies have demonstrated the efficiency of vitamin B6 in curing of nonverbal (NVP) [17]. According to this study, the dual-release composition of doxylamine & B6 improves lifestyle of women with nausea & vomiting in pregnanc by reducing the duration, severity, and recurrence of NVP, as measured by the PUQE score when pyridoxine (B6) is the only medication used [18]. Additionally, utilizing doxylamine with B6 caused a considerable increase in electrolyte levels, as seen in this study by blood potassium levels relative to the other group that received B6 alone, and this is supported [19]. Ferritin levels significantly increased in the group receiving pyridoxine and doxylamine during the NVP treatment in our study, compared to the group receiving pyridoxine alone, and this is in line with [20].
Conclusion
Together the pyridoxine & doxylamine have shown to be efficient and safe for treating NVP, as well as to improve blood iron and electrolyte levels.
References
- Nelson-Piercy C, Dean C, Shehmar M, et al. The Management of Nausea and Vomiting in Pregnancy and Hyperemesis Gravidarum (Green-top Guideline No. 69). BJOG. 2024;131(7):e1-30.
- Gadsby R, Rawson V, Dziadulewicz E, et al. Nausea and vomiting of pregnancy and resource implications: the NVP Impact Study. Br J Gen Pract. 2019;69(680):e217-e223.
- Gadsby R, Ivanova D, Trevelyan E, et al. Nausea and vomiting in pregnancy is not just ‘morning sickness’: data from a prospective cohort study in the UK. Br J Gen Pract. 2020;70(697):e534-e539.
- Birkeland E, Stokke G, Tangvik RJ, et al. Norwegian PUQE (Pregnancy-Unique Quantification of Emesis and nausea) identifies patients with hyperemesis gravidarum and poor nutritional intake: a prospective cohort validation study. PloS one. 2015;10(4):e0119962.
- Yildiz G, Mat E, Kurt D, et al. Hyperemesis Gravidarum and Its Relationship with Placental Thickness, PAPP-A, and Free Beta-HCG: A Case-Control Study. South Clin Istanb Eurasia. 2022;33(4).
- Kirthan JA, Somannavar MS. Pathophysiology and management of iron deficiency anaemia in pregnancy: a review. Ann Hematol. 2024;103(8):2637-2646.
- Dande A, Pajai S, Gupta A, et al. Unraveling the Role of Maternal Serum Ferritin Levels in Preterm Delivery: A Comprehensive Review. Cureus. 2024;16(2).
- Yang CW, Li S, Dong Y. The Prevalence and Risk Factors of Hypokalemia in Pregnancy-Related Hospitalizations: A Nationwide Population Study. Int J Nephrol. 2021;2021(1):9922245.
- Erick M, Cox JT, Mogensen KM. ACOG practice bulletin 189: nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(5):935.
- Yang CW, Li S, Dong Y. The Prevalence and Risk Factors of Hypokalemia in Pregnancy-Related Hospitalizations: A Nationwide Population Study. Int J Nephrol. 2021;2021(1):9922245.
- Schleußner E, Jäkel S, Keck C, et al. Nausea and Vomiting of Pregnancy and its Management with the Dual-Release Formulation of Doxylamine and Pyridoxine. Geburtshilfe Frauenheilkd. 2024;84(02):144-152.
- Marasli M, Keles E, Yildiz P, et al. Clinical Importance of the First Trimester Uterine Artery Doppler Measurements in Patients with Hyperemesis Gravidarum.
- d’Hauterive SP, Close R, Gridelet V, et al. Human chorionic gonadotropin and early embryogenesis. Int J Mol Sci. 2022;23(3):1380.
- Madendag Y, Madendag IÇ, Sahin ME, et al. ILK TRIMESTERDE CIDDI HIPEREMEZIS GRAVIDARUMUN PAPP-A VE HCG ÜZERINE ETKISI Effect of Severe Hyperemesis Gravidarum on PAPP-A and hCG in First Trimester. Bozok Tip Dergisi. 2019;9(4):32-35.
- Fejzo MS, Trovik J, Grooten IJ, et al. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat Rev Dis Primers. 2019;5(1):62.
- Slaughter SR, Hearns-Stokes R, van der Vlugt T, et al. FDA approval of doxylamine–pyridoxine therapy for use in pregnancy. N Engl J Med. 2014 Mar 20;370(12):1081-1083.
- Mousavi-Hasanzadeh M, Adelnia A, Farokhmehr G, et al. The effect of Vitamin B6 on chemotherapy induced nausea and vomiting in pediatric cancer. Iran J Pediatr Hemat. 2020 Jan 5.
- Koren G, Vrandrick M. VM Treating symptoms of morning sickness: the first dual release combination of doxylamine-pyridoxine. Int J Pharm. 2018;8(3):52-58.
- Jayawardena R, Majeed S, Sooriyaarachchi P, et al. The effects of pyridoxine (vitamin B6) supplementation in nausea and vomiting during pregnancy: a systematic review and meta-analysis. Arch Gynecol Obstet. 2023;308(4):1075-1084.
- Li T, Zhang J, Li P. Ferritin and iron supplements in gestational diabetes mellitus: less or more?. Eur J Nutr. 2024;63(1):67-78.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Widad Abd AL-Jabbar1*, Nada Abdulhur Abed Alibraheemi2, Yasmeen Ali Hussein2,3, Saad Mashkoor Waleed4 and Najah R. Hadi52Department of Pharmacology and Therapeutics, College of Medicine, University of Kufa, Iraq
3ARCPMS, University of Al Kafeel, Najaf, Iraq
4Department of Pharmacology, College of Pharmacy, University of Al Kafeel, Najaf, Iraq
5Department of Physiology, College of Pharmacy, University of Al Kafeel, Najaf, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.