Research - (2025) Volume 20, Issue 2
Factors affecting outcome of frozen-thawed embryo transfer cycles in Al-Sadder fertility centre in AL-Najaf AL-Ashraf
Batool Abdulwahid Hashim AlkhalidiReceived: 21-Apr-2025, Manuscript No. gpmp-25-162476; Editor assigned: 23-Apr-2025, Pre QC No. P-162476; Reviewed: 05-May-2025, QC No. Q-162476; Revised: 23-Jun-2025 Published: 30-Jun-2025
Abstract
Background: Owing to the worldwide rising interests in embryo freezing and the adoption of thawed Frozen Embryos Transfer (TFET) in preference to Fresh Cycles Embryo Transfer (FET) by Assisted Reproduction (ART) practitioners in a step that thought to bypass the unwanted effects of exaggerated rise in estradiol level of fresh cycles on endometrium, developing embryos, and placentas, and as preventive measure to Ovarian Hyperstimulation Syndrome (OHSS) consequences especially in polycystic ovary syndrome patients, This study was conducted in fertility centre to retrospectively analyze embryos vitrification technology in our province regarding factors affecting the outcome of FTET. Aim: To study factors affecting outcomes of frozen thawed embryos transfer cycles in it is recent introduction in our center. Design: Retrospective study of all thawed frozen embryos transfer cycles done in our center since the technology was first introduced there. Setting: Kufa University, medical college -affiliated fertility center. Population of patients: all patients treated by thawed frozen embryos transfer cycles Outcomes measured: Biochemical pregnancy rate. Patients and method: A retrospective observational study was conducted in the Fertility Centre in Al sadder medical city in Najaf province in Iraq, a cohort of 47 FTET cycles were collected during the period from April 2014 to June 2016 patients were included having their records reviewed retrospectively, the following demographic criteria were collected; age, Body Mass Index (BMI), cause, type and duration of infertility, antecedent cycle criteria for each patient which include Cycle Day 2 (CD2) hormonal assay, infertility, antecedent cycle criteria for each patient which include Cycle Day 2 (CD2) hormonal assay, type of protocol for Controlled Ovarian Hyperstimulation( COH), number of retrieved oocytes, maturity, and quality of oocytes, fertilization rate, in transfer cycles of FTE embryo numbers, stage after thawing and clinical pregnancy rate. All patient seen at cycle day 2 or 3 transvaginal confirmed down regulated ovarian endometrial cycles, endometrial builtup with oral estradiol, when desired thickness achieved, progesterone added for a period that match day of embryos age. The patients were tested using serum B-Human Chorionic Gonadotrophin (B-HCG) assay 14 days after embryo transfer, and if the pregnancy test was positive, prescription of estradiol valerate (6mg per day) and progesterone in form of vaginal suppositories (800mcg per day) was continued until 11 weeks of gestation. When the embryo transfer catheter returned back to the embryologist after the procedure, it is carefully checked under the microscope to confirm catheter clearance off. Embryo at vetrification. Embryo transfer is usually done in our center without anesthesia, guided by transabdominal sonography with partially filled urinary bladder, When the embryo transfer catheter returned back to the embryologist after the procedure, it is carefully checked under the microscope to confirm catheter clearance, serum pregnancy test routinely done 2 weeks following FTET, if test positive for pregnancy luteal phase support would continue throughout 1st trimester. Results: A total of 47 women who underwent embryo cryopreservation were all involved. Their biochemical pregnancy rate was 35% calculated for 40 patients who reached embryo transfer (in 4 patients no valid embryo to transfer after thawing, and in 3 patients no information was obtained about pregnancy result). Demographic information (patients age, BMI, type of infertility, duration of infertility) and frozen thawed embryo transfer cycle criteria (endometrial thickness, number of transferred embryos, stage of transferred embryos) of all of them were studied and interpreted. Significant differences were found for body mass index (P value 0.002) and endometrial thickness (P value 0.004 ) in predicting pregnancy positivity. Lower body mass index and thicker endometrium were noted in positive pregnancy group. Moreover, there is a significant difference in number of expanded blastocyst stage between the two groups with 78.6% of them found to have a positive pregnancy test (P <0.001).
Keywords
Frozen embryos transfer; Fresh cycles embryo transfer; Reproduction; Fertility; Pregnancy
Introduction
Since the first successful Frozen Thawed Embryo Transfer (FTET) which was resulted in life birth in 1983 [1]. Embryo cryopreservation became an integral part of assisted reproduction service all over the world rebelling all initial safety concerns regarding perinatal and maternal outcome [2,3], the growing evidence around the last 7 years or around supported the equal benefits or even the preponderance of FTET over Fresh Embryo Transfer (FET) in all or at least in certain situations during In Vitro Fertilization (IVF)/ Intracytoplasmic Sperm Injection (ICSI) treatment regarding endometrial receptivity [4] and perinatal outcomes [5].
Up to date, main proved advantages of the procedure is to store surplus embryos thus reducing the chance of multiple births, elective freezing of all embryos in high risk patients of severe Ovarian Hyperstimulation Syndrome (OHSS), thin endometrium patients or other uterine factors and patients who are candidates for Preimplantation Genetic Diagnosis (PGD) [6-9], the number of FTET cycles has dramatically increased over the last years, In the United States, 86,266 FET cycles were performed in 2016, resulting in approximately 33,000 live births [10], in countries where Single Embryo Transfer (SET) were adopted, the proportion of FET treatments approaching 80% [11], in Iraq, the technique of FTET was relatively only recently introduced, and it was first introduced in our centre in Al-Najaf Al-Ashraf city, the first pregnancy conceived by ICSI achieved in this centre in January 2010, however it was only in 2015, the delivery of the first Iraqi baby born from cryopreserved embryos who was a male baby born in 23th of August 2015 happened [12], this study was conducted in our centre to assess factors that are affecting the outcome of Intra-Cytoplasmic Sperm Injection (ICSI) when the embryo/s transferred is/are frozen –thawed.
Patients, Method and Material
In our center the birth of the first neonate conceived by FTET in a governmental center all over the country, who was named Ali at the 23rd of August 2015. In the next month another baby girl was born called Noor AL-Zahraa. This management development has stopped wastage of embryos that were not transferred at the fresh cycle and led to cryopreserving them for further attempts of pregnancy. This retrospective observational study was conducted in the Fertility Centre in Al sadder medical city in Najaf province in Iraq. It is a teaching center belongs to Najaf Health Directorate. Cohorts of 47 FTET cycles were collected. All patient underwent FTET during the period from April 2014 to June 2016 patients were included. Confidentiality was considered in keeping their privacy. Seven cases were excluded, 4 of them had their embryos being not suitable for transfer after thawing, while the other 3 escaped follow up and remained with unknown outcomes. The following demographic criteria were collected; age, Body Mass Index (BMI), cause, type and duration of infertility, antecedent cycle criteria for each patient which include Cycle Day 2 (CD2) hormonal assay, type of protocol for Controlled Ovarian Hyperstimulation (COH), number of retrieved oocytes, maturity, and quality of oocytes, fertilization rate, embryo number, stage and clinical pregnancy rate. Serum pregnancy tested 14 days after FTET.
Patients were seen at cycle day 2 normal size ovaries and thin endometrium were confirmed for each by transvaginal ultrasound examination, endometrium build up was accomplished by hormone replacement therapy by gradually increasing the dose of estradiol valerate in form of 2mg oral tablets. In each follow up visit, transvaginal ultrasound measurement of endometrial thickness is done until it becomes suitable for the addition of progesterone which was added for 2-5 days according to the developmental stage of stored embryos. The route of progesterone given in our center is a combination of both vaginal, intramuscular and oral. Embryo transfer is usually done in our center without anesthesia, guided by transabdominal sonography with partially filled urinary bladder, embryologist hand over the embryo/embryos -loaded soft transfer catheter to the gynecologist to do the transfer. When the embryo transfer catheter returned back to the embryologist after the procedure, it is carefully checked under the microscope to confirm transfer.
The patients were tested using serum B-Human Chorionic Gonadotrophin (B-HCG) assay 14 days after embryo transfer, and if the pregnancy test was positive, prescription of estradiol valerate (6mg per day) and progesterone in form of vaginal suppositories (800mcg per day) was continued until 13 weeks of gestation.
Results and Discussion
A total of 44 infertile women had been included in this study. Their mean age was 29.8±5.79 years (range 20-42 years). There were 14 women of them, who had positive pregnancy test, and 26 had negative pregnancy test and in 4 of them no embryo transfer was performed.
In this retrospective study we analyzed the outcome of cycles after frozen-thawed embryo transfers and also the clinical and embryological factors affecting the pregnancy outcome of embryo cryopreservation in it is recent years of it is first introduction.
Over duration from April 2014 to June 2016 according to the records of the fertility Centre, total of 47 infertile women who undergone Frozen-Thawed Embryo Transfer (FTET) had been included in this study Fig. 1. Their mean age was 29.88±5.79 years (range 20-42 years). Their mean BMI was 25.59±3.57 (range 19-34). Mean duration of infertility was 7.59±3.70 years (range 2-18 years) as shown in Tab. 1.
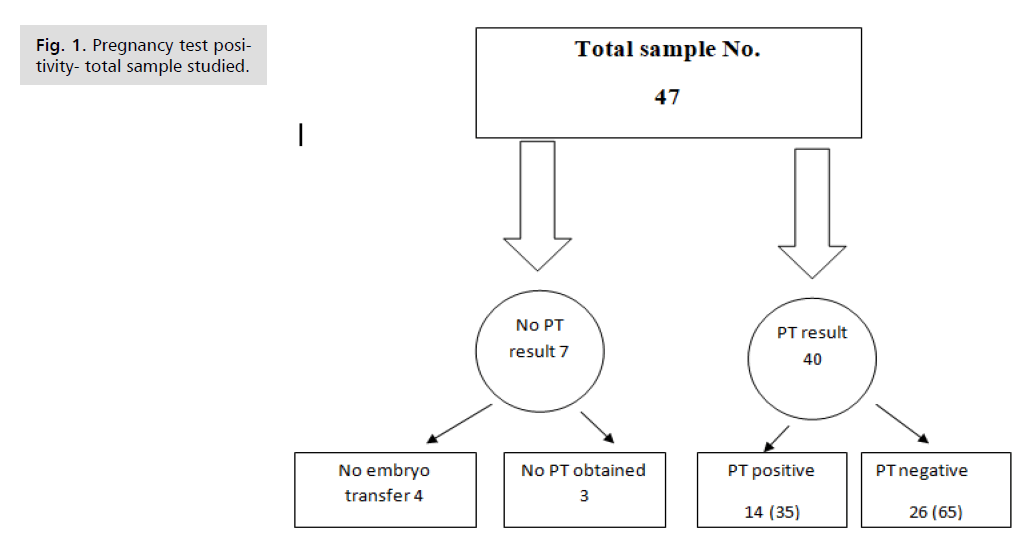
Fig. 1. Pregnancy test positivity- total sample studied.
| N | Minimum | Maximum | Mean | Std. Deviation | ||
|---|---|---|---|---|---|---|
| Age/Years | 44 | 20 | 42 | 29.8864 | 5.7918 | |
| BMI Kg/m2 | 44 | 19 | 34 | 25.5909 | 3.57197 | |
| Duration of infertility/Years | 44 | 2 | 18 | 7.5909 | 3.70617 | |
| Type of Infertility | Number | Percentage | ||||
| Primary | 37 | 84.1 | ||||
| Secondary | 7 | 15.9 | ||||
| Total | 44 | 100 | ||||
Tab. 1. Demographic characteristics of studied women.
Our data showed that the overall biochemical pregnancy rate after frozen – thawed embryo was 35% which is comparable to that of Veleva, et al. [13] whose pregnancy rate rate was 31.3%, while higher than that of Ashrafi, et al. [14] and Van der Elst, et al. [15] ( 28.1% and 27.3% respectively), but lagging much behind Shapiro, et al. [16] whose clinical pregnancy rate per transfer was 84.0% in the cryopreservation group vs. 54.7% in the fresh group.
In our study and as shown in Tab. 2., BMI was found to be a significant predictor for positive pregnancy test i.e., with each 1kg/m2 increment in BMI there is 51% reduction of positive pregnancy test. These results disagree with those of Veleva, et al. [13], who stated that BMI was associated with only minimal decrease in live birth rate. This probably can be explained by the fact that 40% of our patients were overweight or obese as shown in Fig. 2. More recently Zhang, et al. [17] study revealed that obesity worsen pregnancy rate after FTET possibly by affecting endometrial receptivity. However, Prost, et al. [18] study showed no statistically significant difference in live birth rate between lean and obese women having FTET. These contradictory results need further evaluation.
| PT | N | Mean | Std. Deviation | Std. Error Mean | P value | |
|---|---|---|---|---|---|---|
| Age/Years | P | 14 | 29 | 4.97687 | 1.33012 | 413 |
| N | 26 | 30.6538 | 6.51117 | 1.27695 | ||
| BMI Kg/m2 | P | 14 | 23.2857 | 2.64367 | 0.70655 | 0.002 ¥ |
| N | 26 | 26.9615 | 3.59423 | 0.70489 | ||
| Duration/Years | P | 14 | 7.7143 | 3.70921 | 0.99133 | 0.914 |
| N | 26 | 7.5769 | 3.89022 | 0.76294 | ||
| FSH (IU/L) | P | 14 | 5.00071 | 2.383874 | 0.637117 | 0.764 |
| N | 26 | 4.76769 | 2.286698 | 0.448458 | ||
| LH (IU/L) | P | 14 | 2.5229 | 0.94138 | 0.25159 | 0.139 |
| N | 26 | 3.21 | 1.54702 | 0.3034 | ||
| PRL (ng/ml) | P | 14 | 16.4279 | 7.60495 | 2.03251 | 0.341 |
| N | 26 | 19.6819 | 11.28028 | 2.21224 | ||
| E2 at time of ovum pickup (pg/ml) | P | 14 | 2619.214 | 749.8302 | 200.4005 | 0.002 ¥ |
| N | 26 | 1727.654 | 844.4537 | 165.611 | ||
| Oocytes No. | P | 14 | 17.2 | 7 | 1.8 | 0.008 ¥ |
| N | 26 | 11.8 | 5 | 1.009 | ||
| Mature oocytes No. | P | 14 | 12.21 | 6.97 | 1.86 | 0.015 ¥ |
| N | 26 | 7.69 | 4.23 | 0.829 | ||
| Fertilized oocytes No. | P | 14 | 8.3571 | 4.65101 | 1.24303 | 0.028 ¥ |
| N | 26 | 5.5385 | 3.11424 | 0.61075 | ||
| Fertilization rate% | P | 14 | ¥71.36 | 16.653 | 4.451 | 0.987 |
| N | 26 | 71.23 | 26.06 | 5.111 | ||
| Good quality embryos No. | P | 14 | 2.9286 | 0.61573 | 0.16456 | 0.901 |
| N | 26 | 3 | 2.07846 | 0.40762 | ||
| Endometrial thickness (mm) | P | 14 | 13.1429 | 1.83375 | 0.49009 | 0.004 ¥ |
| N | 26 | 10.3846 | 3.12508 | 0.61288 |
Tab. 2. Basic IVF cycle characteristics of studied women.
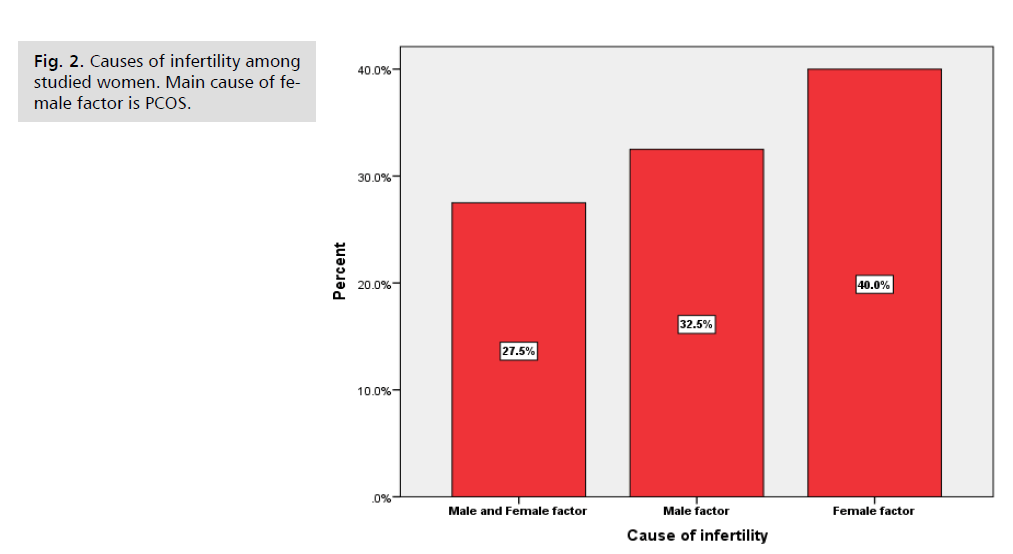
Fig. 2. Causes of infertility among studied women. Main cause of female factor is PCOS.
In Tab. 2., the mean level of E2 at time of ovum pick up in positive pregnancy group was 2619.2 pg/ml while it was 1727.6 pg/ml in the negative pregnancy group (P value 0.002) which puts the E2 as a significant predictor for positive PT as it is proportional to higher number of retrieved oocytes.
There was a significant difference in oocytes number, mature oocytes and fertilized oocytes between the two groups where all of them were higher in positive group. The mean number of retrieved oocytes was 17.2 in those with positive PT in comparison to 11.8 in those with negative PT (P value 0.008). This can be explained by increasing the availability of embryos to be preserved and transferred in the next cycle, However, analysis of data of Ashrafi, et al. [14] disagrees with these results as the number of retrieved oocytes has a converse impact on the b-HCG result according to his/her study. He found that with the increase of number of oocytes, the chance of pregnancy decreases owing to reduced oocytes quality.
Another important factor which is taken in consideration in our study is the quality of embryo transferred. The classification of embryo morphology and the growth rate of embryos were observed by using noninvasive method to assess the developmental potential of human preimplantation embryos. Poor classification of embryo morphology is often associated with chromosomal abnormality.
High quality criteria include several factors that have previously been reported to affect the outcome: good morphology at freezing [19-21], lack of damage at thawing [20,21] and resumed cleavage after overnight culture [22-24]. The processes of freezing and thawing damaged embryos, but the extent of this damage varies from one embryo to another. Tab. 2. in our data shows that the mean number of good quality embryos was 2.9286± 0.61573 in patients PT positive result, while it was 3.0000 ±2.07846 in patients with negative PT with no statistically significant difference (P value 0.901). This may be explained by the fact that in our center the embryos that were preserved and transferred were only of good quality. Previous studies have shown that only 30–48% of embryos survive cryopreservation intact [23] and Sole, et al. [21]. However, top quality embryos seem to do better in Veleva, et al. [13] study where in 79% of the cycles with top quality embryos frozen, at least one embryo still had high quality morphology after thawing, indicating that top quality embryos are more viable than embryos of poorer quality. While Salumets, et al. [20] have, in fact reported significantly higher pregnancy rates in those who had higher quality of embryo transfer (Grade A: regular cleavage, no fragmentation or Grade B: regular /slightly irregular, less than 20% fragmentation, cell dark) than in those with poor-quality embryo transfer.
Selick, et al. [25] evaluated the effect of cryopreservation on embryo quality and pregnancy outcome of embryos in fresh and frozen ova from the same donor and discovered that cryopreservation has a detrimental effect on embryo quality, but the rates of pregnancy are not impaired after transfer of embryos. Our study did not support the idea of freezing all embryos for transfer in FTET in order to improve the outcomes. This was in agreement with Shapiro, et al. [16] and Maheshwari and Bhattacharya [26] who preferred cryopreservation of only good quality embryos. Furthermore, Veleva, et al. [13] study suggested that live birth rate in the group with no top quality embryos frozen was quite low (10.4%). This further supports our trend in cryopreservation of only good quality embryos to avoid costly treatments with a low success rate.
The mean measurement of endometrial thickness (mean ET) in those with biochemical pregnancy (positive BhCG titer) was 13.14±1.83, while in those with negative pregnancy test, the mean ET was 10.38±3.125, as shown in Tab. 3. (P value is 0.004) with increase of ET by 1mm there is a 52% increase chance of positive pregnancy test making this factor to be a significant predictor of biochemical pregnancy.
| B | S.E. | Wald | df | P value | Odds ratio Exp (B) | 95% C.I. for OR Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age | 0.193 | 0.134 | 2.053 | 1 | 0.152 | 1.212 | 0.932 | 1.578 |
| BMI | -0.712 | 0.308 | 5.357 | 1 | 0.021 | 0.491 | 0.268 | 0.897 |
| Duration | -0.267- | 0.216 | 1.529 | 1 | 0.216 | 0.765 | 0.501 | 1.169 |
| FSH (IU/L) | -0.833- | 0.504 | 2.726 | 1 | 0.099 | 0.435 | 0.162 | 1.169 |
| LH (IU/L) | 1.622 | 0.896 | 3.277 | 1 | 0.07 | 5.065 | 0.875 | 29.33 |
| PRL(ng/ml) | -0.053- | 0.079 | 0.449 | 1 | 0.503 | 0.948 | 0.812 | 1.108 |
| E2 (pg/ml) | 0.582 | 0.001 | 5.21 | 1 | 0.022 | 2.342 | 1.109 | 3.721 |
| Endometrial thickness (mm) | 0.422 | 0.212 | 3.947 | 1 | 0.047 | 1.525 | 1.006 | 2.311 |
| No. of embryo transfer | -2.114 | 1.43 | 2.231 | 1 | 0.135 | 0.117 | 0.007 | 1.953 |
Tab. 3. Binary logistic regression for different predictors for positive pregnancy (Adjusted odds ratio).
Endometrial thickness is a major factor in identifying the time of progesterone supplementation as well as the time of frozen thawed embryo transfer. In the previous studies performed by EL-Toukhy, et al. [27], Noyes, et al. [28] and Zhang, et al. [29], who evaluated the relationship between endometrial thickness and treatment outcome after frozen thawed embryo transfer, a significantly higher pregnancy rate in women with endometrial thickness greater than or equal to 9 mm on HCG or embryo transfer day in comparison with that in women with an endometrial thickness of 7-8 mm. In the study of Ashrafi, et al. [14] none of the women with endometrial thickness of less than 8 mm became pregnant. While in Check, et al. [30] study, no difference was found in pregnancy outcome in women with endometrial thickness of less than or equal to 8 mm in comparison with those having endometrial thickness of 9-14 mm.
Mahutte, et al. [31] found that live birth rate decreased dramatically if ET was less than 6 mm. Nevertheless, Shaodi, et al. [32] study revealed that the best outcome was when ET was in the range of 8.7-14.5 mm and that endometrium of less or more than this range was associated with reduced live birth rate.
The day of cryopreservation of the embryo also had an impact on the positive pregnancy results. We found that day 5 and day 6 transferred embryos had 50.0% positive PT result (P value 0.036). Twelve from the fourteen ladies, who get pregnant in our study, have their embryos cryopreserved at day 5 and 6. The development of stage–specific sequential culture media systems which support the blastocyst stage has made it possible to delay embryo transfer until day 5\6. The aim of extended culture is to produce blastocysts with better implantation potential than cleavage-stage embryos. Transfer of embryos at day 5 can eliminate those embryos that are unable to develop after activation of the zygote genome due to genetic or metabolic defects. As shown in Tab. 4. there is a significantly higher pregnancy rate in the transfer of full expansion staged embryos i.e. (11 from the 14 positive result while only 1 for each early blastocyst stage, 4 cell stage and 8 cell stage embryos (P value ≤ 0.001). Current studies suggest that blastocyst transfer has a place in the treatment of selected groups of patients, in particular those with a high risk of multiple gestations or for whom a multiple pregnancy represent an obstetrical disaster. In patients who develop three or more good quality eight- cell stage embryos on day 3, extended culture to the blastocyst stage has achieved implantation rates in the order of 40%, with pregnancy rate of at least 65% according to Gardner, et al. [33] which was comparable to our study.
| PT | Total | P value | |||
|---|---|---|---|---|---|
| N | P | ||||
| Type of infertility | Primary | 21 | 12 | 33 | 0.695 |
| 63.60% | 36.40% | 100.00% | |||
| Secondary | 5 | 2 | 7 | ||
| 71.40% | 28.60% | 100.00% | |||
| Cause of infertility | Male and female factors | 7 | 4 | 11 | 0.916 |
| 63.60% | 36.40% | 100.00% | |||
| Male factor | 8 | 5 | 13 | ||
| 61.50% | 38.50% | 100.00% | |||
| PCOS | 11 | 5 | 16 | ||
| 68.80% | 31.20% | 100.00% | |||
| Protocol of COH | antagonist | 18 | 8 | 26 | 0.483 |
| 69.20% | 30.80% | 100.00% | |||
| Long | 1 | 0 | 1 | ||
| 100.00% | 0.00% | 100.00% | |||
| Short | 7 | 6 | 13 | ||
| 53.80% | 46.20% | 100.00% | |||
| Embryo stage | 4 cells | 5 | 1 | 6 | <0.001 |
| 83.30% | 16.70% | 100.00% | |||
| 8 cells | 8 | 1 | 9 | ||
| 88.90% | 11.10% | 100.00% | |||
| early blastocyst | 10 | 1 | 11 | ||
| 90.90% | 9.10% | 100.00% | |||
| Expanded blacystocyst | 3 | 11 | 14 | ||
| 21.40% | 78.60% | 100.00% | |||
| Day of embryo vitrification | Day 1 | 2 | 1 | 3 | 0.036 |
| 66.70% | 33.30% | 100.00% | |||
| Day 2 or 3 | 12 | 1 | 13 | ||
| 92.30% | 7.70% | 100.00% | |||
| Day 5 or 6 | 12 | 12 | 24 | ||
| 50.00% | 50.00% | 100.00% | |||
| No. of embryo transfer | 03-Apr | 12 | 13 | 25 | 0.004 |
| 48.00% | 52.00% | 100.00% | |||
| 01-Feb | 14 | 1 | 15 | ||
| 93.30% | 6.70% | 100.00% | |||
| Total | 26 | 14 | 40 | ||
| 65.00% | 35.00% | 100.00% | |||
Tab. 4. Association between positive and negative groups in categorical variables.
In our study, number of embryo transfer had significant association with PT result where 13 women of the total 14 who had positive PT were had 3-4 embryos transferred as shown in Tab. 4. There is a controversy among studies regarding the relationship between the numbers of embryos.
Andersen, et al. [34], Van deer Elsa, et al. [15], Laveran, et al. [35] and Oehninger, et al. [36] agree and have reported a higher pregnancy rate with greater number of embryos transferred while Hyden-Granskoget, et al. [37] fail to prove this relationship. Wang, et al. [38] found that pregnancy rate for single frozen thawed embryo transfer was 10% -14%. The expectation of pregnancy for two embryos could be more than 20% and the risk of multiple births was 15%-29% according to his study. By increasing the number of embryos, clinical pregnancy rates and multiple birth rates increased significantly. In the light of goal of increasing the pregnancy rate and multiple birth rate, it is recommended that before deciding how many embryos should be transferred, patient’s general condition and the quality of embryos should be taken into consideration.
Important factor affecting the outcome of FTET is the method of cryopreservation used in our Centre i.e. vitrification which is considered efficient and simple and eliminates the extra cellular ice formation preventing cryo- injury that may damage the embryo.
The most important factors amongst all are the skills, efficiency and well trained team who work harmonically that led to this result.
Conclusion
The rate of the biochemical pregnancy after FTET in our center is 35%, normal BMI, higher serum E2 level at time of ovum pickup and endometrial thickness are the significant predictors for positive PT, higher number of retrieved oocyte, number of mature oocytes, number of fertilized oocytes and fertilization rate all were associated with higher biochemical pregnancy rate. Increasing number of embryos transferred, day 5 embryo developmental stage vitrification also were associated with higher PT positivity, in our study the number good quality embryos was not significantly higher in positive PT group and this may be explained by the cryopreservation of only good quality embryos, The cryopreservation method used in our center is vitrification which is associated with low risk on the viability of embryos, in our brief study, only 4 of 47 couples had no viable embryos after thawing.
References
- Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305(5936):707-709.
- Belva F, Henriet S, Van Den Abbeel E, et al. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod. 2008;23(10):2227-2238.
- Maheshwari A, Pandey S, Amalraj Raja E, et al. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer?. Hum Reprod Update. 2018;24(1):35-58.
- Vuong LN, Dang VQ, Ho TM, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med. 2018;378(2):137-147.
- European IVF-Monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE), Kupka MS, et al. Assisted reproductive technology in Europe, 2011: Results generated from European registers by ESHRE. Hum Reprod. 2016;31(2):233-248.
- Tsai NC, Su YT, Lin YJ, et al. Developmental potential of surplus morulas with delayed and/or incomplete compaction after freezing-thawing procedures. Reprod Biol Endocrinol. 2019;17:1-8.
- Shin JJ, Jeong Y, Nho E, et al. Clinical outcomes of frozen embryo transfer cycles after freeze-all policy to prevent ovarian hyperstimulation syndrome. Obstet Gynecol Sci. 2018;61(4):497.
- Costello MF, Garad RM, Hart R, et al. A review of second-and third-line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med Sci. 2019;7(7):75.
- Wang X, Wu H, He X, et al. Retrospective study to compare frozen-thawed embryo transfer with fresh embryo transfer on pregnancy outcome following intracytoplasmic sperm injection for male infertility. Med Sci Monit. 2018;24:2668.
- https://www.cdc.gov/art/pdf/2016-national-sum%20mary-slides/ART_2016_graphs_and_charts.pdf
- European IVF-monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE), et al. Assisted reproductive technology in Europe, 2013: Results generated from European registers by ESHRE. Hum Reprod. 2017;32(10):1957-1973.
- Rahim AI, Al-Kawaz UM, Abdulla TH. Although Late; but the First, an Iraqi Success in Human Embryo Cryopreservation Using Vitrification and the Factors Affecting the Pregnancy Rate: Cross-Sectional Study. Iraqi J Embryos Infertil Res. 2018;8(1).
- Veleva Z, Orava M, Nuojua-Huttunen S, et al. Factors affecting the outcome of frozen–thawed embryo transfer. Hum Reprod. 2013;28(9):2425-2431.
- Ashrafi M, Jahangiri N, Hassani F, et al. The factors affecting the outcome of frozen–thawed embryo transfer cycle. Taiwan J Obstet Gynecol. 2011;50(2):159-164.
- Van der Elst J, Van den Abbeel E, Camus M, et al. Endocrinology: Long-term evaluation of implantation of fresh and cryopreserved human embryos following ovarian stimulation with buserelin acetate-Human Menopausal Gonadotrophin (HMG) or clomiphene citrate-HMG. Hum Reprod. 1996;11(10):2097-2106.
- Shapiro BS, Daneshmand ST, Restrepo H, et al. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99(2):389-392.
- Zhang J, Liu H, Mao X, et al. Effect of body mass index on pregnancy outcomes in a freeze-all policy: An analysis of 22,043 first autologous frozen-thawed embryo transfer cycles in China. BMC Med. 2019;17:1-9.
- Prost E, Reignier A, Leperlier F, et al. Female obesity does not impact live birth rate after frozen-thawed blastocyst transfer. Hum Reprod. 2020;35(4):859-865.
- Hartshorne GM, Wick K, Elder K, et al. Effect of cell number at freezing upon survival and viability of cleaving embryos generated from stimulated IVF cycles. Hum Reprod. 1990;5(7):857-861.
- Salumets A, Suikkari AM, Mäkinen S, et al. Frozen embryo transfers: Implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod. 2006;21(9):2368-2374.
- Sole M, Santalo J, Rodriguez I, et al. Correlation between embryological factors and pregnancy rate: Development of an embryo score in a cryopreservation programme. J Assist Reprod Genet. 2010;28:129-136.
- Ziebe S, Bech B, Petersen K, et al. Resumption of mitosis during post-thaw culture: A key parameter in selecting the right embryos for transfer. Hum Reprod. 1998;13:178-181.
- Guerif F, Bidault R, Cadoret V, et al. Parameters guiding selection of best embryos for transfer after cryopreservation: a reappraisal. Hum Reprod. 2002;17:1321-1326.
- Gabrielsen A, Fedder J, Agerholm I. Parameters predicting the implantation rate of thawed IVF/ICSI embryos: a retrospective study. Reprod Biomed Online. 2006;12:70-76.
- Selick CE, Hofmann GE, Albano C, et al. Embryo quality and pregnancy potential of fresh compared with frozen embryos: Is freezing detrimental to high quality embryos? Hum Reprod. 1995;10:392-395.
- Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: Ready for prime time? Hum Reprod. 2013;28:6-9.
- El-Toukhy T, Coomarasamy A, Khairy M, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89:832-839.
- Noyes N, Liu HC, Sultan K, et al. Endometrial thickness appears to be a significant factor in embryo implantation in in vitro fertilization. Hum Reprod. 1995;10:919-922.
- Zhang X, Chen CH, Confino E, et al. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2005;83:336-340.
- Check JH, Dietterich C, Graziano V, et al. Effect of maximal endometrial thickness on outcome after frozen embryo transfer. Fertil Steril. 2004;81:1399-1400.
- Mahutte N, Hartman M, Meng L, et al. Optimal endometrial thickness in fresh and frozen-thaw invitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. 2022;117(4):792-800.
- Shaodi Z, Qiuyuan L, Yisha Y, et al. The effect of endometrial thickness on pregnancy outcomes of frozen-thawed embryo transfer cycles which underwent hormone replacement therapy. PLoS One. 2020;15(9):e0239120.
- Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF. Hum Reprod Update. 1997;3:367-382.
- Andersen AN, Gianaroli L, Felberbaum R, et al. The European IVF-monitoring programme (EIM), European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2001. Results generated from European registers by ESHRE. Hum Reprod. 2005;20:1158-1176.
- Levran D, Dor J, Rudak E, et al. Pregnancy potential of human oocytes: The effect of cryopreservation. N Engl J Med. 1990;323:1153-1156.
- Oehninger S, Mayer J, Muasher S. Impact of different clinical variables on pregnancy outcome following embryo cryopreservation. Mol Cell Endocrinol. 2000;27(169):73-77.
- Hyden-Granskog C, Unkila-Kallio L, Halttunen M, et al. Single embryo transfer is an option in frozen embryo transfer. Hum Reprod. 2005;20:2935.
- Wang XJ, Ledger W, Payne D, et al. The contribution of embryo cryopreservation to in-vitro fertilization/gamete intrafallopian transfer: 8 years' experience. Hum Reprod. 1994;9:103-109.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Batool Abdulwahid Hashim AlkhalidiCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.