Research - (2023) Volume 18, Issue 1
Efficacy of intraperitoneal Lidocaine for post cesarean section Analgesia: A prospective randomized, double-blind, placebo- controlled clinical trial
Sarah Hamada Mohamed Hamza*Received: 14-Aug-2022, Manuscript No. gpmp-22-71881; Editor assigned: 17-Aug-2022, Pre QC No. P-71881; Reviewed: 29-Aug-2022, QC No. Q-71881; Revised: 20-Mar-2023, Manuscript No. R-71881; Published: 29-Mar-2023
Abstract
Aim: To investigate the effect of intraperitoneal instillation of lidocaine on post-cesarean delivery pain.
Patients and Methods: This study is a Prospective, Randomized, Double blinded, Placebo-controlled Clinical trial, was carried out at Ain shams university hospital Obstetrics and Gynecology department, on (200) women divided into:2 groups: (Group I) (Lidocaine group): comprised 100 women who received 20ml of 2% lidocaine with epinephrine (1:200,000, (Group II) (Placebo group) which is the (COTROL GROUP): comprised 100 women who received 20ml normal saline, during the time period from 1st of August 2021to the end of Novamber 2021.
Results: The pain scores are significantly lower in the Lidocaine group at 4 hours (mean difference=14.8, 95% CI=13.0 to 16.6, P-value <0.0001), 6 hours (mean difference=17.6, 95% CI=15.7 to 19.5, P-value <0.0001), and 12 hours (mean difference=18.8, 95% CI=17.2 to 20.4, P-value <0.0001). Test of within-subjects effects shows a statistically significant effect of time (F=2303.090, df= 2, P value < 0.001) with a statistically significant Group * Time interaction (F=24.620, df= 2, P value < 0.001) Stabbing pain and tension-type pain were more common in the Control group (14% vs. 2%, p-value=0.002 and 8% vs. 0%, P-value=0.007, respectively). On the other hand, burning pain was more common in the Lidocaine group (26% vs. 7%, P-value < 0.001) Significantly fewer patients required supplemental analgesic in the Lidocaine group compared with the Control group (35% vs. 92%, respectively, P-value < 0.001). The consumption of paracetamol, diclofenac sodium and nalbuphine as well as the cumulative analgesic consumption are all significantly less in the Lidocaine group (all P-values < 0.001). Fewer patients in the Lidocaine group complained of nausea (23% vs. 48%, P-value < 0.001). The incidence of vomiting was comparable in both groups (P-value=0.171).
Conclusion: Intraperitoneal instillation of 20 ml of 2% lidocaine with epinephrine (1:200.000) decreased the post cesarean pain scores and the for opioids, is easy to use with high safety margin, does not require, and is cost effective.
Keywords
Intraperitoneal lidocaine; Cesarean section; Analgesia
Introduction
Cesarean section (CS) is a surgical procedure in which one or more incisions are made through a mother’s abdominal layers and uterus to deliver one or more babies. A CS is supposed to be performed when a vaginal delivery would put the baby’s or mother’s life or health at risk [1].
The Cesarean Delivery is at a rate of 52 percent in Egypt which stands out among countries with the highest CS delivery rates in the world, following Dominican Republic (56.4 percent) and Brazil (55.6 percent) [2].
Women with severe acute postpartum pain were found to have a 2.5-fold increased risk of persistent pain and a 3.0-fold increased risk of postpartum depression compared with those with mild postpartum pain [3].
Postoperative pain frequently has nociceptive characteristics, that is, it derives from tissue or organ lesions, whose nociceptive stimuli are perceived as painful [4].
Pain is usually classified into two main categories by the type of damage that causes it. These two categories are nociceptive and neuropathic pains. During surgery, tissue injury causes nociceptive pain and nerve damage causes neuropathic pain [5].
Postoperative pain relief is very important issue in any surgery. The common postoperative pain management is traditionally based on opioids. Considering opioids’ adverse effects such as nausea vomiting, sedation, drowsiness, and urinary retention, recently there is a lot of interest for finding a safe and effective pain treatment after operation. Several new methods are introduced for postoperative pain relief [6].
Local anesthetics are recognized as a useful technique for postoperative pain management. They can reduce inflammatory response after surgery and produce analgesia by blocking neural transmission at the site of tissue injury [7].
A suitable local anesthetic should be effective, safe, and inexpensive. Lidocaine is a proper and the most widely used local anesthetic. Lidocaine is used in several ways for managing the postoperative pain [8].
The ease of use and safety of intraperitoneal local anesthetics (IPLA) are well recognized, and the main advantage is that they are not associated with the adverse effects of systemically administered opioids. Their use as an effective adjunct in postoperative multimodal analgesia has been reported for decades in laparoscopic gastric procedures, gynecological surgery, and open abdominal surgery, including open abdominal hysterectomy [9].
Aim of the Work
The aim of the study is to investigate the effect of intraperitoneal instillation of lidocaine on post-cesarean delivery pain.
Patients and Methods
Type of study: Prospective, Randomized, Double blinded, Placebo-controlled Clinical trial.
Study setting: The study was conducted at Ain Shams University hospital Obstetrics and Gynecology department.
Study approval: The study was approved by research ethical committee, Faculty of Medicine, Ain Shams University.
Study duration: The study was conducted during the time period from 1st of August 2021(data collection) to the end of November 2021(analysis of data & its results).
Study population: Women were recruited from outpatient obstetric clinic, antenatal clinic, and obstetric department inpatients. Two hundred (200) women were recruited to this study, which were randomly divided into:
Group I (Lidocaine group): It comprised 100 women who received 20ml of 2% lidocaine with epinephrine (1:200,000).
Group II (Placebo group) which is the (COTROL GROUP): It comprised 100 women who received 20ml normal saline.
Sample size justification:
Using PASS 11 program, based on a study (Patel, et al.), sample size of 200 patients (100 in treatment arm and 100 in control arm) was sufficient in VAS score to detect difference of 10 mm and achieve power of 80% and confidence level of 95%. The common SD in both groups is 24mm. Sample size was inflated by 15% to account for attrition problem in prospective studies.
Inclusion criteria: Women undergoing cesarean section under spinal anaesthesia.
Exclusion criteria:
• Women undergoing cesarean section under general anesthesia whether upon patient’s request, or due to any contraindication for spinal anesthesia or due to obstetric emergency that interferes with the operation time & outcome.
• Use of regional anesthesia other than spinal (epidural, trans vs. abdominis plane block, Quadratus lumborum block, etc.) as it affected our pain scoring.
• Neurological comorbidities (chronic pain disorders, chronic systemic diseases, neurological disorders) as they needed more analgesics.
• Pregnancy induced medical disorders (hypertension, gestational diabetes mellitus, any neurological complications, etc.) as they needed intensive care for assessment and management.
• Drug abuse. These patients usually are very tolerant to the substance they use. Therefore, pain control can be achieved only with substantially higher doses of opioids.
• Allergy of either of the drugs used in the study which was tested by an expert nurse before surgery.
• BMI ≥40 as they were not suitable for spinal anesthesia.
• Intraoperative adhesions which are more than fibrous band will block our field and take more time for dissection.
Any intraoperative complications will occur during caesarean section (bowel or bladder injuries, bleeding, blood transfusion, etc.) that will affect our outcome.
Study Procedures and methodology: All patients underwent cesarean section with spinal anesthesia. This surgery and the procedure were done through my 3 supervisors and experienced surgeons with master's degrees. The time of surgery was within one hour (skin incision to closure of abdominal wall).
Methodology:
Patient information: We explained details of the procedure, aim of the work, benefit and risk of the trial to all patients.
Patient consent: Informed consent was obtained from all participants (written consent).
Method of randomization: After obtaining written informed consent, the surgeon randomized the women before cesarean section into 2 groups using, sealed identical envelopes containing the word intra-peritoneal instillation of Lidocaine into their peritoneal cavity or Placebo) that will be opened before closure of the fascia.
Women were assigned numbers based on the order of inclusion criteria in the study hence the order of receiving the operation, A randomization of 200 into 2 groups using computer generated table of randomization was done, where group 1 received 20 ml of 2% lidocaine with epinephrine (1:200,000) and group 2 received 20 ml normal saline (a placebo drug).
A sample size of 200 patients (n= 100 each for 2 groups) was calculated using PASS 11 program. A total 220 patients recruited; 10 patients excluded based on exclusion criteria, 3 patients refused the study and 7 patients had failed spinal anaesthesia (Fig. 1.).
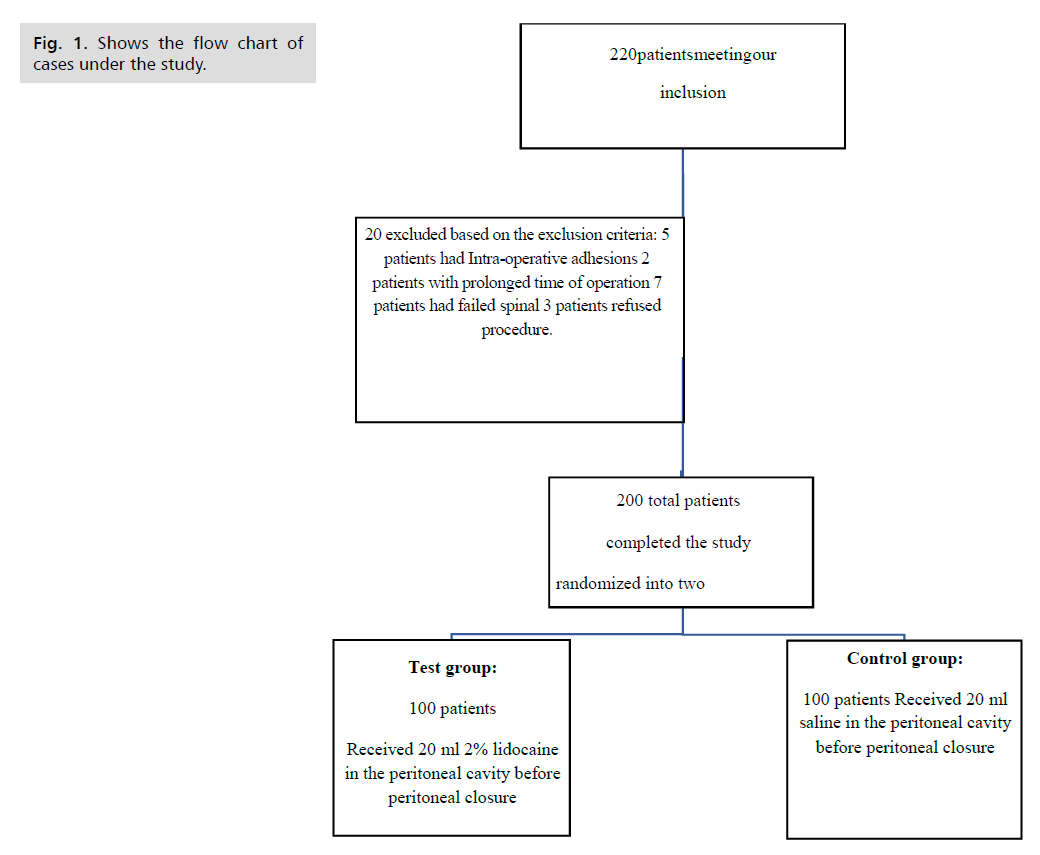
Fig 1. Shows the flow chart of cases under the study.
Method of blinding: The study drug was prepared in a sterile manner under supervision of anesthesiologist who was not involved in clinical evaluation of trial outcomes. The randomization code was not broken and analyzed except after the study was concluded. The patient and obstetric resident following up the patients were blinded to the content of injected solution. The operating surgeon was aware of the study protocol and the injected solution.
History taking, examination, and investigations: After obtaining informed consent patients were informed by the anesthesiologist about the postoperative analgesia protocol. All women were told that they would be receiving acetaminophen and diclofenac regularly and that opioid analgesia would be reserved for breakthrough pain and given upon the patient’s request. Patients were instructed on the use of a 100-mm visual analogue scale (VAS, 0=no pain and 100=worst pain imaginable), where they would be exposed to regular rotations by the research, 6 and 12 hours postoperative to collect VAS data from the patients, the blank line measuring 100 mm would be shown to the patients they marked a point on the line in between the no pain and worst pain imaginable ends of the scale and it was measured and noted in millimeters compared to previous assessments. The paten was asked to describe the type of pain, wither it was (colicky/visceral – somatic/superficial).
Operative details:
Standardized spinal anesthetic will be administered, consisting of 2 mL of 0.5% hyperbaric bupivacaine, 25 μgfentanyl. Standardized cesarean section will be conducted to all patients: Pfannenstiel incision with removal of previous scar if present, combined sharp and blunt dissection of the anterior abdominal wall, lower segment C shaped incision of the uterus will be performed.
• After delivery of fetus and placenta, closure of uterus in 2 layers will be done by vicryl 0 on round needle, the blood accumulating into the pelvis will be carefully wiped with surgical towels to leave a relatively dry pelvis. After good hemostasis, either the study drug or nomal saline was transferred into a sterile receptacle and drawn up into a sterile 20-mL syringe without a needle. Then carefully instillation of the study drug or 20 ml normal saline on to the uterine peritoneal area by spraying 5 mL of the drug or normal saline onto each quadrant of the uterus before closure of the parietal peritoneum or fascia. The parietal peritoneum layer will be sutured or left opened according to operator preference, sheath closure all with running suture using Vicryl 1 suture on cutting needle, closure of campers fascia using simple interrupted sutures, finally closure of skin using running subcuticular suture using Vicryl 0 suture on cutting needle.
• The study dose of 20 ml of 2% lidocaineis not toxic and was used before in previous study, (Patel, et al.).
• The lidocaine used in the study was purchased by the research doctor, the study was self-funded.
Pain management protocol:
i. World Health Organization (WHO) Stepwise, Multimodal Approach were implemented to all patients [10].
• Step one includes non-opioid analgesics;
1. Nonsteroidal anti-inflammatory drugs: diclofenac sodium 75mg amp/12 hours starting 3 hours post-operative.
2. Acetaminophen: paracetamol 2 tablets 500mg/6 hours starting 4 hour's post-operative.
3. Step two when pain cannot be adequately managed with step one non-opioid medications milder opioids will be introduced. Nalbuphine 20 mg amp diluted in 9 ml normal saline, a 3 ml slow IV administration
1. Nausea treated with ondansetron, metoclopramide or dimenhydrinate.
2. Medications were provided by the hospital as routine to all cesarean section patients.
Post-operative assessment by a study investigator.
The following were being assessed:
• Pain at 4, 6 and 12 hours postoperative using visual analog scale (VAS).
• Nausea and vomiting.
• Shoulder pain
• Mobility onset, frequency and pain with mobility will be assessed.
• Return of intestinal function (passing of flatus or stool).
• Need for first dose of analgesia and total use of analgesia.
Primary outcome:
VAS pain scores at 6 hours after cesarean delivery.
Secondary outcome:
Effect of lidocaine on;
• Maternal pain score at 4 hours and 12 hours, post operatively
• Nausea and vomiting
• Shoulder pain.
• Mobility.
• Intestinal motility.
• Consumption of opioids.
Ethical considerations: The study was started after approval of Research ethics committee, the faculty of medicine, Ain Shams University. Informed consent will be taken from all participants before recruitment in the study and after explaining the procedure.
Certificate of consent:
I have read the foregoing information, or it has been read to me. I have had the opportunity to ask questions about it and any questions that I ask have been answered to my satisfaction. I consent voluntary to participate in
this research and understand that I have the right to withdraw from the research at any time without in any way affecting my patient`s medical care.
• Name of participant:
• Signature of legal guardian/or participant:
• Identity number or finger print: …………………
• Date:
I have accurately read or witnessed the accurate reading of the consent to the potential participant. The individual has had the opportunity to ask questions I confirm that the individual has given consent freely.
• Name of researcher: Sarah Hamada Mohamed.
• Signature of researcher:
• Date:
Statistical Methods
Statistical analysis was done using Data were analyzed using IBM© SPSS© Statistics version 26 (IBM© Corp., Armonk, NY) and MedCalc® Statistical Software version 20 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021).
Categorical variables are presented as counts and percentages and intergroup differences are compared using the Pearson chi-squared test. Ordinal data are compared using the chi-squared test for trend.
Numerical variables are presented as mean and standard deviation and intergroup differences are compared with the independent-samples t-test.
Serial measurement analysis is used to calculate summary measures for the pain scores using the methods described by Mathews J, et al. [11]. The area under the time-VAS curve (AUC), time-weighted average TWA) and minimum and maximum VAS scores are calculated and compared between groups using the unpaired t-test.
Repeated measures analysis of variance (ANOVA) is used to examine between-subjects and within-subjects effects as regards the change in pain scores.
P-values <0.05 are considered statistically significant.
Results
Tab. 1. shows the BMI is significantly higher in the Lidocaine group. Tab. 2. shows that there is no statistically difference for obstetrics history in the both study groups. Tab. 3. shows that the pain scores at 4, 6 and 12 hours postoperative are significantly lower in the Lidocaine group. Tab. 4. shows the area under the VAS-Time curve (AUC), time-weighted average VAS (TWA), minimum VAS and maximum VAS are all significantly lower in the Lidocaine group (all P-values <0.0001). The assumption of sphericity is not violated, so no correction was done for the degrees of freedom. Test of between-subjects effects shows a statistically significant between-group difference. Test of within-subjects effects shows a statistically significant effect of time with a statistically significant group * Time interaction (Tab. 5.). Tab. 6. shows that stabbing pain and tension-type pain were more common in the control group, on the other hand, burning pain was more common in the Lidocaine group. Tab. 7. shows that significantly fewer patients required supplemental analgesic in the Lidocaine group compared with the Control group. Tab. 8. shows that fewer patients in the Lidocaine group complained of nausea.
| Variable | Lidocaine group (n=100) | Control group (n=100) | Difference | 95% CI | P-value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 28.4 ± 5.8 | 28.9 ± 6.2 | 0.5 | -1.2 to 2.1 | 0.588† |
| BMI (kg/m2), mean ± SD | 29.4 ± 3.5 | 27.0 ± 2.9 | -2.4 | -3.3 to -1.5 | <0.0001† |
| Smokers, n (%) | 21 (21%) | 21 (21%) | >0.999‡ |
‡. Fisher’s exact test
Tab. 1. Demographic characteristics of both study groups.
| Variable | Lidocaine group (n=100) | Control group (n=100) | P-value† |
|---|---|---|---|
| Number of children, n (%) | 0.226 | ||
| Nil | 24 (24.0%) | 22 (22.0%) | |
| 1 Child | 26 (26.0%) | 27 (27.0%) | |
| 2 Children | 30 (30.0%) | 20 (20.0%) | |
| 3 Children | 13 (13.0%) | 16 (16.0%) | |
| ≥4 Children | 7 (7.0%) | 15 (15.0%) | |
| Frequency of previous CS, n (%) | 0.904 | ||
| Nil | 31 (31.0%) | 35 (35.0%) | |
| 1 CS | 28 (28.0%) | 30 (30.0%) | |
| 2 CS | 27 (27.0%) | 17 (17.0%) | |
| 3 CS | 11 (11.0%) | 11 (11.0%) | |
| 4 CS | 3 (3.0%) | 7 (7.0%) | |
| Frequency of previous NVD, n (%) | 0.034 | ||
| Nil | 86 (86.0%) | 75 (75.0%) | |
| 1 NVD | 5 (5.0%) | 8 (8.0%) | |
| 2 NVD | 5 (5.0%) | 5 (5.0%) | |
| 3 NVD | 2 (2.0%) | 6 (6.0%) | |
| 4 NVD | 2 (2.0%) | 6 (6.0%) |
Tab. 2. Obstetric history in both study groups.
| Variable | Time | Lidocaine group (n=100) | Control group (n=100) | Difference | 95% CI | P-value |
|---|---|---|---|---|---|---|
| VAS, mean± SD | 4 h | 56.4 ± 5.8 | 71.2 ± 6.9 | 14.8 | 13.0 to 16.6 | <0.0001 |
| 6 h | 45.6 ± 6.4 | 63.2 ± 6.9 | 17.6 | 15.7 to 19.5 | <0.0001 | |
| 12 h | 34.6 ± 5.5 | 53.4 ± 5.8 | 18.8 | 17.2 to 20.4 | <0.0001 |
Tab. 3. Pain scores in both study groups.
| VAS | Group | n | Mean | SD | Difference | 95% CI | P-value† |
|---|---|---|---|---|---|---|---|
| AUC | Lidocaine group | 100 | 91.1 | 5.5 | 34.4 | 31.1 to 37.7 | <0.0001 |
| Control group | 100 | 125.5 | 5.8 | ||||
| TWA | Lidocaine group | 100 | 45.6 | 5.5 | 17.2 | 15.5 to 18.8 | <0.0001 |
| Control group | 100 | 62.8 | 6.3 | ||||
| Minimum | Lidocaine group | 100 | 34.6 | 5.5 | 18.8 | 17.2 to 20.4 | <0.0001 |
| Control group | 100 | 53.4 | 5.8 | ||||
| Maximum | Lidocaine group | 100 | 56.6 | 5.7 | 14.8 | 13.0 to 16.5 | <0.0001 |
| Control group | 100 | 71.4 | 6.8 |
AUC=area under the time-VAS curve, TWA=time-weighted average.
Tab. 4. Serial measurement analysis for the change in pain scores.
| Sphericity | |||||
|---|---|---|---|---|---|
| Method | Epsilon | ||||
| Greenhouse-Geisser | 0.992 | ||||
| Huynh-Feldt | 1.000 | ||||
| Test of Between-Subjects Effects | |||||
| Source of variation | Sum of Squares | DF | Mean Square | F | P-value |
| Groups | 43673.602 | 1 | 43673.602 | 437.220 | <0.001 |
| Residual | 19778.023 | 198 | 99.889 | ||
| Test of Within-Subjects Effects | |||||
| Source of variation | Sum of Squares | DF | Mean Square | F | P-value |
| Time | 39357.570 | 2 | 19678.790 | 2303.090 | <0.001 |
| Group* Time interaction | 420.803 | 2 | 210.402 | 24.620 | <0.001 |
| Residual | 3383.627 | 396 | 8.545 | ||
Tab. 5. Repeated measures analysis of variance (ANOVA) for the change in pain scores.
| Variable | Lidocaine group (n=100) | Control group (n=100) | P-value† | |
|---|---|---|---|---|
| Predominant type of pain, n (%) | Shoulder pain | 17 (17.0%) | 17 (17.0%) | 1.000 |
| Colicky pain | 21 (21.0%) | 18 (18.0%) | 0.592 | |
| Stabbing pain | 2 (2.0%) | 14 (14.0%) | 0.002 | |
| Burning pain | 26 (26.0%) | 7 (7.0%) | <0.001 | |
| Epigastric pain | 16 (16.0%) | 15 (15.0%) | 0.845 | |
| Tension-type pains | 0 (0.0%) | 8 (8.0%) | 0.007‡ | |
| Mobilization-related Pain | 18 (18.0%) | 21 (21.0%) | 0.592 |
‡. Fisher’s exact test
Tab. 6. Predominant type of pain in both groups.
| Variable | Lidocaine group | Control group | P-value† |
|---|---|---|---|
| Need for analgesics | 35 (35.0%) | 92 (92.0%) | <0.001‡ |
| Frequency of paracetamol consumption (500-mg PO), n (%) | <0.001 | ||
| Nil | 91 (91.0%) | 80 (80.0%) | |
| 1 Dose | 9 (9.0%) | 0 (0.0%) | |
| 2 Doses | 0 (0.0%) | 10 (10.0%) | |
| 3 Doses | 0 (0.0%) | 10 (10.0%) | |
| Frequency of diclofenac Na consumption (75 mg IM), n (%) | <0.001 | ||
| Nil | 74 (74.0%) | 53 (53.0%) | |
| 1 Dose | 26 (26.0%) | 2 (2.0%) | |
| 2 Doses | 0 (0.0%) | 22 (22.0%) | |
| 3 Doses | 0 (0.0%) | 22 (22.0%) | |
| 4 Doses | 0 (0.0%) | 1 (1.0%) | |
| Frequency of nalbuphine consumption (3 mg IV) , n (%) | <0.001 | ||
| Nil | 100 (100.0%) | 76 (76.0%) | |
| 1 Dose | 0 (0.0%) | 6 (6.0%) | |
| 2 Doses | 0 (0.0%) | 18 (18.0%) | |
| Cumulative frequency of analgesic requests in 24 h, n (%) | <0.001 | ||
| Nil | 65 (65.0%) | 9 (9.0%) | |
| 1 Dose | 35 (35.0%) | 8 (8.0%) | |
| 2 Doses | 0 (0.0%) | 50 (50.0%) | |
| 3 Doses | 0 (0.0%) | 32 (32.0%) | |
| 4 Doses | 0 (0.0%) | 1 (1.0%) |
Tab. 7. Analgesic consumption in both groups.
| Variable | Lidocaine group (n=100) | Control group (n=100) | P-value† | |
|---|---|---|---|---|
| Adverse outcomes, n (%) | Nausea | 23 (23.0%) | 48 (48.0%) | <0.001 |
| Vomiting | 36 (36.0%) | 27 (27.0%) | 0.171 |
Tab. 8. Incidence of adverse outcomes in both groups.
Discussion
Cesarean delivery is a commonly performed procedure worldwide. The cesarean delivery rate is just over 32% in the United States, and with close to 4 million births per year, this results in approximately 1.3 million cesarean deliveries performed per year [12].
Severe acute postpartum pain is associated with chronic pain. Women with severe acute postpartum pain were found to have a 2.5-fold increased risk of persistent pain and a 3.0-fold increased risk of postpartum depression compared with those with mild postpartum pain [3].
Persistent pain and depression can adversely affect maternal–infant interaction and breastfeeding. The ideal method for post cesarean pain relief should be cost-effective, simple, and safe for the mother, providing high-quality pain relief with low incidence of side effects and complications. Also, it should not interfere with the maternal care of the newborn or with the establishment of breast-feeding, and should involve drugs that are minimally excreted into breast milk. Furthermore, immobility from inadequate pain control or sedation from opioids places these women at greater risk of thromboembolic events, delayed recovery and discharge from the hospital [13].
Multimodal analgesia provides synergistic or additive analgesia with fewer side effects and has greatly improved pain control for the majority of women after cesarean delivery. However, there remain a proportion of women for whom postoperative pain relief and patient satisfaction are still inadequate [14].
These women are at increased risk of developing acute severe pain after delivery and the adverse consequences outlined previously. Previous study by Pan PH, et al. [15] has focused on identifying factors to predict post cesarean delivery pain. Stratifying patients at risk for postoperative hyperalgesia and chronic pain and administering tailored multimodal analgesic therapy are recommended.
The ease of use and safety of intraperitoneal local anesthetics (IPLA) are well recognized, and the main advantage is that they are not associated with the adverse effects of systemically administered opioids. Their use as an effective adjunct in postoperative multimodal analgesia has been reported for decades in laparoscopic gastric procedures, cholecystectomy, gynecological surgery, and open abdominal surgery, including open abdominal hysterectomy [16].
A literature search revealed very few studies investigating the use of intraperitoneal local anesthetics for pain management after cesarean delivery. The main aim of this study was to investigate the effect of intraperitoneal instillation of lidocaine on post-cesarean delivery pain.
This Prospective, Randomized, Double blinded, Placebo-controlled Clinical trial, conducted at Ain shams university hospital Obstetrics and Gynecology department and the study was conducted during the time period from 1st of August 2021 to the end of November 2021. In the current study we assessed 220 patients for eligibility to participate in the study. 20 patients either declined or were found ineligible, which resulted in the recruitment of 200 patients into the study. 20 excluded based on the exclusion criteria, 5patients had Intra-operative adhesions, 2 patients with prolonged time of operation, 7 patients had failed spinal and 3 patients refused procedure. The 200 women randomly divided into: Group I (Lidocaine group): 100 women received 20ml of 2% lidocaine with epinephrine (1:200,000) and Group II (Placebo group), which is the (control group): 100 women received 20ml normal saline.
The main results of this study were as following:
As regard demographic characteristics of the studied groups, we found that there is no statistically significant difference between both groups as regards the age. The BMI is significantly higher in the Lidocaine group but the difference is of no clinical value.
The present study was supported by the Randomized, Double-Blind, Placebo-Controlled Trial, by Patel R, et al. [16] who aimed to investigate the effect of intraperitoneal instillation of lidocaine on post cesarean delivery pain. The study enrolled 99 women in Lidocaine group and 94 women as controls, demographic data were similar of both groups.
As well the prospective randomized, double-blind, placebo-controlled study by Anwar MAW, et al. [13] aimed to evaluate intraperitoneal (IP) lidocaine administration and intravenous (IV) lidocaine infusion for postoperative pain control after cesarean section, they enrolled a total of 150 pregnant full-term women divided into three groups control group (n=50), Intraperitoneal group (n=50) and Intravenous group (n=50), there was no statistically significant difference between both groups as regard demographic data.
One more randomized double-blind placebo-controlled Trial by Shahin AY & Osman AM [17] both groups as regard demographic Characteristics.
In our study the obstetric history in both study groups, we found that both groups are comparable as regards the number of children and Frequency of previous CS, as shown in Tab. 2. (P-value=0.226 and 0.904, respectively). The percentage of women in the Lidocaine group that had 1 normal vaginal delivery (NVD), 2 NVD, 3 NVD or 4 NVD is 5%, 5%, 2% and 2%, respectively compared with 8%, 5%, 6% and 6%, respectively, in the Control group. The differences are statistically significant (P-value=0.034).
In agreement with our results Patel R, et al. [16] revealed that there was no statistically significant difference between groups as regard Gravida, parity and Primary cesarean delivery.
Also, Anwar MAW, et al. [13] reported that there was no statistically significant difference between both groups as regard parity.
Furthermore, Shahin AY & Osman AM [17] reported that there was no statistically significant difference between both groups as regard parity as well as indications of Cesarean Section.
Concerning Pain scores in both study groups, the present study revealed that the pain scores are significantly lower in the Lidocaine group as shown in Tab. 3.
The present study also showed that the area under the VAS-Time curve (AUC) (mean difference=34.4, 95% CI=31.1 to 37.7), time-weighted average VAS (TWA) (mean difference=17.2, 95% CI=15.5 to 18.8), minimum VAS (mean difference=18.2, 95% CI=17.2 to 20.4), and maximum VAS (mean difference=14.8, 95% CI=13.0 to 16.5) are all significantly lower in the Lidocaine group (all P-values <0.0001).
The results of repeated measures analysis of variance (ANOVA) for the change in pain scores revealed that the assumption of sphericity is not violated (Greenhouse-Geisser epsilon=0.992, Huynh-Feldt epsilon=1.000), so no correction was done for the degrees of freedom. Test of between-subjects effects shows a statistically significant between-group difference (F=437.220, df=1, 198, P value < 0.001). Test of within-subjects effects shows a statistically significant effect of time (F=2303.090, df= 2, P value < 0.001) with a statistically significant Group * Time interaction (F=24.620, df= 2, P value < 0.001) as shown in Tab. 5.
In agreement with our results Anwar et al., 2016 reported that there were significantly reduced VAS scores in groups Intraperitoneal and Intravenous compared with controls after 4 h; however, in contrast to our results, this difference was not noted after 6, 12, and 24 h. There was significantly lower total pethidine consumption in 24 h, time to ambulation, onset of pain relief, and the need for rescue analgesia in groups Intraperitoneal and Intravenous compared with controls.
Our results were supported by Shahin AY & Osman AM [17] who reported that regarding abdominal pain scores, significantly higher global abdominal VAS on the first postoperative day was reported among control group patients when compared with lidocaine group patients (4.4 ± 1.4, vs. 2.8 ± 1.3, with a range of 2.0 to 7.0 in both groups, P<0.001).
While Patel R, et al., [16] concluded that there was no significant difference in the primary outcome, pain on movement at 24-hour post cesarean delivery, in patients receiving intraperitoneal lidocaine. They also reported significantly lower pain scores at 2-hour post cesarean delivery and significantly fewer women requesting opioid analgesia for postoperative pain in the lidocaine group compared with placebo. In patients undergoing parietal peritoneum closure, the use of intraperitoneal lidocaine lowered pain scores at 24-hour post-cesarean delivery.
Several studies reported that IP lidocaine at the end of surgery was associated with lower postoperative pain scores after laparoscopic cholecystectomy (400 mg lidocaine) [18], minor laparoscopic gynecological procedures, and total abdominal hysterectomy (200 mg lidocaine) [19].
The previous studies not reported that AUC the VAS curve, TWA-VAS, minimum and the maximum VAS scores.
As regard our study predominant type of pain in both groups, the current study showed that Stabbing pain and tension-type pain were more common in the Control group (14% vs. 2%, p-value=0.002 and 8% vs. 0%, P-value=0.007, respectively), On the other hand, burning pain was more common in the Lidocaine group (26% vs. 7%, P-value < 0.001) as shown in Tab. 6.
While Shahin AY & Osman AM [17] reported that Pain in the different regions were significantly higher in control group than lidocaine group, at 15-days and 6-months (P<0.001). Control group showed significantly higher number of patients reporting of global abdominal pain compared with lidocaine patients on the first postoperative day (P<0.001), 15-days (P<0.001) and after 8 months (P<0.05). More controls complained of epigastric pain compared with lidocaine patients on the first postoperative day (P<0.01), 15-days (P<0.01) and after 8 months (P<0.001). Significantly more control patients reporting of wound pain on the first postoperative day, compared with lidocaine patients (P<0.001). Wound pain persisted more frequently up to 15 days in control group patients (P=0.09). After 8 months, more patients from the control group reported wound pain (P<0.05). In conclusion the study reported that Patients reporting pain 8 months after surgery had significantly lower pain scores on day 1, day 15, and 8 months after surgery when they received intraperitoneal lidocaine, compared with control patients.
In the study on our hands significantly fewer patients required supplemental analgesic in the Lidocaine group compared with the Control group (35% vs. 92%, respectively, P-value < 0.001). The consumption of paracetamol, diclofenac sodium and nalbuphine as well as the cumulative analgesic consumption are all significantly less in the Lidocaine group (all P-values < 0.001) as shown in Tab. 7.
In agreement with our results Shahin AY & Osman AM [17] reported that the use of lidocaine was associated with a significant morphine sparing effect, as evident by significantly few patients requiring morphine injections, less morphine consumption, and less reported side effects in lidocaine group. They also reported that the incidence of persistent post cesarean pain after parietal peritoneal closure has dropped significantly from 20.8% to 10.8% when intraperitoneal lidocaine instillation was used. In their earlier study they suggested that parietal peritoneal closure was responsible for the high persistent post-cesarean pain incidence (25.5%) compared with non-closure technique (10.4%).
While the findings by Patel R, et al. [16] reported that the total amount of morphine (mg, median [interquartile range]) needed for breakthrough pain was not significantly different between the lidocaine and placebo groups: 0 mg [0, 0] in both groups at 2 hours; 0 mg [0, 0] and 2 mg [0, 6], respectively, at 24 hours; 10 mg [10, 20] and 10 mg [0, 10], respectively, at 48 hours. However, the number of women requesting opioids for breakthrough pain within 48 hours after cesarean delivery was significantly lower in the lidocaine group compared with that of the placebo group (40 [40%] vs 61 [65%], respectively, relative risk 0.59 [95% CI 0.43–0.81]; P=.001).
Our results were supported by Anwar MAW, et al. [13] who revealed that there was significantly lower total pethidine consumption in 24 h, time to ambulation, onset of pain relief, and the need for rescue analgesia in groups Intravenous and Intraperitoneal compared with controls.
Also, we found in our study that fewer patients in the Lidocaine group complained of nausea (23% vs. 48%, P-value < 0.001). The incidence of vomiting was comparable in both groups (P-value=0.171).
This can be explained by the lower analgesic consumption among lidocaine patients, resulting into less nausea, vomiting, itching, drowsiness, and earlier mobility, as evidenced by our results. This culminates into an overall better patient satisfaction, less depression and anxiety, and, consequently, better tolerability of the somatic pain element as well. Patients with higher levels of anxiety and depression experience increased pain scores [20].
Also, the study by Anwar MAW, et al. [13] reported that Postoperative nausea and vomiting were less frequently noted in groups intraperitoneal and intravenous than in control group, but this trend was not statistically significant.
As well Shahin AY & Osman AM, [17] reported that morphine side effects, such as nausea, vomiting, drowsiness, and itching were more significantly reported among controls, compared with lidocaine groups (P<0.001) as the lidocaine groups consumed significantly less Morphine amount.
Furthermore, the study by Hirmanpour A, et al. [21] reported that the frequency of the incidence of nausea and vomiting had a significant difference between both groups (p< 0.05). The frequency of the incidence of side effects had no significant difference between both groups (p> 0.05). While the study by Patel et al., 2017 reported that the maternal VAS satisfaction score was not significantly different between both study groups. They also found no significant difference in the incidence of nausea, vomiting, and itching or recovery of bowel function between the two studied groups.
Finally, as regard to our study, Kaplan-Meier curves showed that the Median time to analgesic request=3 hours in the Control group but is not calculable in the Lidocaine group (<50% of patients requested analgesic till 24 hours). Difference between both KM curves is statistically significant (Log rank chi-squared=126.383, df =1, P-value <0.0001). Hazard ratio=0.088, 95% CI=0.058 to 0.135.
We also found that Median time to mobilization=3 hours in the Lidocaine group vs. 6 hours in the Control group. Difference between both KM curves is statistically significant (Log rank chi-squared=141.032, df =1, P-value <0.0001). Incidence rate ratio=12.008, 95% CI=7.967 to 18.097.
And we also found that Median time to resumption of intestinal function=6 hours in the Lidocaine group vs. 7 hours in the Control group. Difference between both KM curves is statistically significant (Log rank chi-squared=10.158, df =1, P-value=0.0014). Incidence rate ratio=1.742, 95% CI=1.238 to 2.450.
To our knowledge this is the first study demonstrating the Kaplan-Meier curves for comparing the time of analgesic request, time to resumption of intestinal function and time to mobilization between Lidocaine group and Placebo group.
Our results were supported by Ghenaee MM, et al. [9] as they reported that the mean interval between the completion of the operation and need to use diclofenac suppository between two groups are compared in Tab. 2. The mean interval between the completions of the operation and need to use diclofenac suppository was significantly less in lidocaine group (p=0.016).
Furthermore, the study by Hirmanpour A, et al. [21] reported that the mean of consuming extra doses of Diclofenac suppository, at the moment of entering the recovery room, 15 and 60 minutes into the recovery and 4 and 12 hours after the surgery was significantly lower in the Lidocaine receiving group compared to the placebo group; their difference was significant (p < 0.05). Considering the injection of extra doses of Pethidine, at the moment of entering the recovery room and 15 minutes into the recovery room and also 4 hours after the surgery, it was lower in the Lidocaine receiving group compared to the placebo group and their difference was significant (p < 0.05).
However, in contrast to this result the study by Anwar MAW, et al. [13] revealed that no significant differences were noted between the groups as regards the time to bowel sounds, the time to starting a regular diet, or the period of hospital stay, although the values were lower in group IV compared with the other two groups
Conclusion
Intraperitoneal instillation of 20 ml of 2% lidocaine with epinephrine (1:200.000) decreased the post cesarean pain scores and for opioids, is easy to use with high safety margin, dose not require, and is cost effective.
Authors Contribution
(A) Study Design · (B) Data Collection · (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Glasier A, Gülmezoglu AM, Schmid GP, et al. Sexual and reproductive health: A matter of life and death. The Lancet. 2006;368(9547):1595-607.
- Betrán AP, Ye J, Moller AB, et al. The increasing trend in caesarean section rates: Global, regional and national estimates: 1990-2014. PloS one. 2016;11(2):e0148343.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140(1):87-94.
- Borges NC, e Silva BC, Pedroso CF, et al. Postoperative pain in women undergoing caesarean section. Enferm Glob. 2017;16(4):374-83.
- Sorouri ZZ, Milani F, Heidarzadeh A, et al. Intraperitoneal instillation of lidocaine for postoperative pain relief after total abdominal hysterectomy: A double blinded randomized placebo-controlled trial. Iran J Pharm Res. 2020;19(2):317.
- Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87(1):62-72.
- Barreveld A, Witte J, Chahal H, et al. Preventive analgesia by local anesthetics: The reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg. 2013;116(5):1141.
- Khan MR, Raza R, Zafar SN, et al. Intraperitoneal lignocaine (lidocaine) vs. bupivacaine after laparoscopic cholecystectomy: Results of a randomized controlled trial. J Surg Res. 2012;178(2):662-669.
- Ghenaee MM, Rahmani S, Jafarabadi MI. Local lidocaine 2% in postoperative pain management in cesarean delivery. J Family Reprod Health. 2015;9(1):19.
- American College of Obstetricians and Gynecologists. Postpartum pain management. ACOG Committee opinion no. 742. Obstet Gynecol. 2018;132(1):e35-43.
- Matthews J, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. Br Med J. 1990;300(6719):230-235.
- Births; Final Data for 2014. National Center for Health Statistics. 2014;64.
- Anwar Murad AW, Elhadi Farag MA, Abosrie M, et al. Efficacy of intraperitoneal vs. intravenous lidocaine for postcesarean pain relief. Evid Based Women's Health J. 2016;6(4):144-148.
- Faboya A, Uncles D. Post Caesarean delivery pain management: Multimodal approach. Int J Obstet Anesth. 2007;16(2):185-186.
- Pan PH, Tonidandel AM, Aschenbrenner CA, et al. Predicting acute pain after cesarean delivery using three simple questions. Anesthesiology. 2013;118(5):1170-1179.
- Patel R, Carvalho JC, Downey K, et al. Intraperitoneal instillation of lidocaine improves postoperative analgesia at cesarean delivery: A randomized, double-blind, placebo-controlled trial. Anesth Analg. 2017;124(2):554-559.
- Shahin AY, Osman AM. Intraperitoneal lidocaine instillation and postcesarean pain after parietal peritoneal closure: A randomized double blind placebo-controlled trial. Clin J Pain. 2010;26(2):121-127.
- Yang SY, Kang H, Choi GJ, et al. Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. J Int Med Res. 2014;42(2):307-319.
- Perniola A, Fant F, Magnuson A, et al. Postoperative pain after abdominal hysterectomy: A randomized, double-blind, controlled trial comparing continuous infusion vs patient-controlled intraperitoneal injection of local anaesthetic. Br J Anaesth. 2014;112(2):328-336.
- Haythornthwaite JA, Menefee LA, Heinberg LJ, et al. Pain coping strategies predict perceived control over pain. Pain. 1998;77(1):33-39.
- Hirmanpour A, Talakoub R, Mansouri H. Effect of intravenous infusion of lidocaine on pain Reduction after cesarean section under general anesthesia. J Cell Mol Anesth. 2018;3(1):22-30.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Sarah Hamada Mohamed Hamza*Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.