Research Article - (2022) Volume 0, Issue 0
Effect of vitamin D oral supplementation in women with clomiphene citrate resistant polycystic ovarian syndrome (A one-arm clinical trial)
Al Hassan Mohammad Khedr, Rana Alaa Eldin*, Hesham Harb and Laila Aly FaridReceived: 17-Jul-2022, Manuscript No. gpmp-22-69377; Editor assigned: 18-Jul-2022, Pre QC No. P-69377; Reviewed: 28-Jul-2022, QC No. Q-69377; Revised: 02-Aug-2022, Manuscript No. R-69377; Published: 29-Sep-2022
Abstract
Background: Some data suggested that vitamin D ameliorates responsiveness to clomiphene citrate (CC) in women with polycystic ovary syndrome (PCOS). Aims: To assess the effect of oral supplement of vitamin D in patients with CC-resistant PCOS.
Subjects and methods: This was conducted in infertility clinic in Ain Shams Maternity Hospital including 130 women who were diagnosed as having PCOS: age of 18 to 37, body mass index of 25 to 35, being CC-resistant (failure to ovulate with 3 months of usage of CC at 150 mg /day for 5 days). The duration of the study ranged from 12 months, Results: As regard to ovulation-post-treatment, 29 (29%) patients could ovulate. As regard to pregnancy rate, 12 (12%) patients were positive and 88 (88%) patients were negative.
Conclusion: We cannot conclude whether this treatment actually improved pregnancy rate; however, some fraction of CC “resistant” women became “responsive”. Controlled study is needed to confirm the result.
Keywords
PCOS; Vitamin D; Clomiphene citrate resistance; Pregnancy; Ovulation; Post-treatment
Introduction
Clomiphene citrate, an anti-Estrogenic drug, is the primary therapy used for ovulation induction in women with the poly cystic ovary syndrome. However, obese women with the syndrome often require multiple courses and high doses of clomiphene, and there is a positive correlation between obesity and the dose of clomiphene required to induce ovulation. Since increasing obesity is associated with increasing hyperinsulinemia, the high degree of hyperinsulinemia in obese women with the polycystic ovary syndrome may account for their poor responsiveness to clomiphene [1].
Numbers of studies have demonstrated associations between vitamin D levels and various PCOS symptoms, including insulin resistance, infertility and hirsutism. Vitamin D is thought to influence the development of PCOS through gene transcription, and hormonal modulation influences insulin metabolism and fertility regulation [2].
It has been shown that HOXA10 expression which is essential for endometrial development is up-regulated by 1,25 (OH)2D3 in human endometrial stroma cells. Vitamin D3 and calcium repletion might lead to normalization of menstrual cycles and restoration of ovulation through oocyte activation and maturation [3].
Vitamin D supplementation can lower the abnormally elevated serum AMH levels in vitamin D-deficient women with PCOS [4].
It is postulated that the excess of AMH in PCOS women decreases the sensitivity of antral follicles to FSH and hence results in follicular arrest, Wong et al. (2018) found that in women with PCOS, serum anti-mullerian hormone levels were positively and independently correlated with the vitamin D levels [5].
Multiple studies have illustrated an inverse association between the vitamin D status, and hyperandrogenism and insulin resistance. Consequently, intervention with vitamin D at doses as high as 50,000 to 60,000 IU/week have provided improvements in the hyperinsulinemia, and androgenic and fertility factors in PCOS women. Overall, high dose vitamin D supplementation has shown promising results in improving the treatment of the PCOS patients [6].
Patient & Methods
Type of study
A one-arm clinical trial. (NCT04916925)
Study setting
Study population: This study included 130 women who are:
• PCOS in child bearing age (from 18 to 37 year old )
• With body mass index from 25 to 35 kg/m2
• Clomiphene citrate resistant that means failure to ovulate with 3 months of usage of CC at 150 mg /day for 5 days [7].
Study place: It was done at infertility clinic at Ain Shams Maternity Hospital.
Time and duration: The study started from August 2019 and ended August 2020.
Sample size justification: Using PASS II program for sample size calculation
The primary outcome was the ovulation rate. A previous study reported that the ovulation rate in clomiphene-resistant PCO patients was 10%. If they received clomiphene only for induction of ovulation [7].
Assuming ovulation rate improved after treatment by 75%, sample size of 130 patients , with 30 % of cases lost during follow up , achieving 100% power to detect this difference with 0.05 significance [8].
Inclusion criteria
1. Age: 18-37 years
2. Polycystic ovarian syndrome diagnosis made according to ESHRE/ASRM criteria.
• Polycystic ovaries (12 or more follicles and increased ovarian volume >10 cm3).
• Oligo-ovulation or anovulation.
• Clinical and/or biochemical signs of hyperandrogenism [9]
3. PCOS infertile women resistant to CC for 3 cycles.
Exclusion criteria
Causes of infertility other than PCOS:
1. Male factor (normal semen analysis, according to WHO criteria 2010) [10]
2. Other factors e.g. endometriosis
3. Tubal factor (normal hysterosalpingography)
4. Endometrial factors: endometritis
5. Uterine factors: adenomyosis, myomas, mullerium dysgnesis.
6. Premature ovarian failure: Day 3 FSH > 14 mu/ml or AMH <1 ng/ml.
7. Causes of anovulation other than PCOS.
8. Patients with hyperprolactinemia.
9. Patients with thyroid dysfunction.
10. Current or recent use of Vit D treatment.
Interventions and treatment
• History taking
Taking a proper history from the patient:
● Personal history: age, duration of marriage, occupation
● Medical history: any previous or current medical conditions
● Surgical history: abdominal or pelvic surgery
● Drug history: drugs taken for any chronic disease
● Menstrual history: menarche, duration, regularity, amount
● Family history: history of chronic or previous diseases
Examination
General examination
● Especially breast examination for galactorrhea
● Thyroid examination for thyroid dysfunctions.
Local examination
● Palpation: if there is any palpable masses or tender abdomen
● Pelvic examination of uterus and ovaries by bimanual examination and by ultrasound
Investigations
FSH & AMH
Interventions: In this trial 130 clomiphine citrate resistant PCOS pateints, (clomiphene citrate resistance means failure to ovulate with 3 months from usage of clomid at 150 mg/day for 5 days), FSH & AMH were
measured for each patient.
They received vitamin D (ossofortin®, Eva Pharma) orally 10,000 IU twice weekly for three months [11] with clomiphene citrate (Clomid®50 mg tablet, Global Napi pharmaceuticals) orally 150 mg/day for 5 days. Folliculometery started at day 9 of the cycle and every other day till dominant follicle was observed (= >18 ml in diameter) or till day 21 of cycle for 3 successive cycle.
Patients were given HCG 10,000 IU IM (choriomon® 5000 IU vial, IBSA) then timed sexual intercourse was recommended from 36 to 48 hours after taking the injection. Then trans-vaginal ultrasound was done. Free fluid in douglus pouch and decrease in size of follicle (corpus luteum cyst) was noted. Then, B-HCG was done 2 weeks after giving the trigger to assess pregnancy rate [12].
Primary outcome: Ovulation rate: which is defined as, the average number of released follicles in a group of patients [13].
Secondary outcome:
• Biochemical pregnancy rate: [Time Frame: serum B-HCG 2 weeks after HCG injection] [14].
• Clinical pregnancy rate: trans-vaginal ultrasound confirmation of a gestational sac.
• Evaluation of the endometrial thickness at day of trigger [Time Frame: in cycle day 14 and 21]
Statistical Methods
Data was analyzed using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY). Normally distributed numerical data was presented as mean and SD, and skewed data as median and interquartile range. Qualitative data was presented as number and percentage. Comparison of normally distributed numerical data was done using the paired t test. Skewed data was compared using the Mann-Whitney test. Categorical data was compared and the appropriate statistical test was done. P-value <0.05 was considered statistically significant.
Data was expressed as mean ± standard deviation or number (%).
All patients entering the trial were counselled and were sign a written consent explaining the details of the trial. The study was done on 130 patients with eligible criteria but 30 of them were lost in follow up thus the study was done on 100 patients only (Fig. 1.).
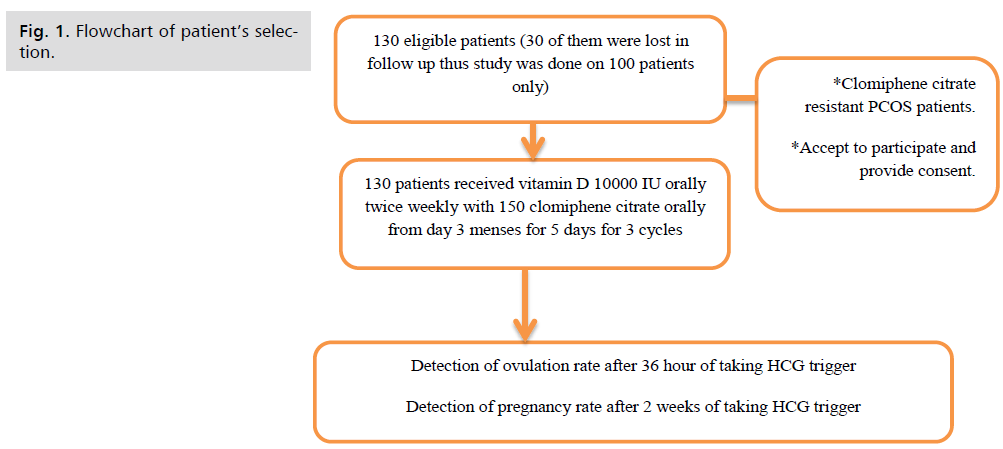
Fig 1. Flowchart of patient’s selection.
Results
Age of the patients ranged from 25-37 years, mean value 31.86 ± 3.5 years (Tab. 1.).
Period of infertility ranged from 2-7 years, mean value 4.78 ± 1.16 years (Tab. 1.).
There were 82 (82%) patients with primary infertility and 18 (18%) patients with secondary infertility (Tab. 1.).
There were 26 (26%) patients with family history of PCOS and 20 (20%) patients with early menarche (Tab. 1.).
The duration of symptoms ranged from 1-12 years with a median value 6 years (Tab. 1.).
| variables | Patients (n = 100) |
|
|---|---|---|
| Age (years) |
Mean ± SD | 31.86 ± 3.5 |
| Range | 25-37 | |
| Period of infertility | Mean ± SD | 4.78 ± 1.16 |
| Range | 2-7 | |
| Parity | P0 | 82 (82%) |
| P1 | 18 (18%) | |
| Type of infertility | Primary | 82 (82%) |
| Secondary | 18 (18%) | |
| Family history of PCOS | 26 (26%) | |
| AMH | Mean ± SD | 8.17 ± 3.45 |
| Range | 3.0 – 15.0 | |
| FSH | Mean ± SD | 5.08 ± 1.84 |
| Range | 2.20 – 8.11 | |
| Endometrial thickness at day of ovulation | Mean ± SD | 6.54 ± 0.90 |
| Range | 5.70 – 8.90 | |
Tab. 1. Patients' characteristics of all studied patients.
As regard to pregnancy rate each month, 3rd month showed higher pregnancy rate (7% chemical pregnancy & 3% clinical pregnancy) than 1st month (2% chemical & 1% clinical) and 2nd month ( 3% chemical &1% clinical ) (Tab. 2.).
| Pregnancy Rate | Patients (n = 100) | |
|---|---|---|
| 1st month | chemical | 2(2.0%) |
| clinical | 1(1.0%) | |
| 2nd month | chemical | 3(3.0%) |
| clinical | 1(1.0%) | |
| 3rd month | chemical | 7(7.0%) |
| clinical | 3(3.0%) | |
Tab. 2. Monthly pregnancy rate of all studied patients.
t: Student t-test
• As regard to ovulation rate in 1st month, 7 (7%) pateints ovulated whose:
• Age ranged from 26- 34 years with a mean value 30.29 ± 2.63 (Tab. 3.).
• BMI ranged from 25.3 - 31.8 kg/m2 with a mean value 27.97 ± 3.0 (Tab. 3.).
• AMH ranged from 5.0 – 11.0 ng/ml with a mean value 7.57 ± 2.3.0 (Tab. 3.).
• Endometrial thickness ranged from 5.7-8.8 mm with a mean value 6.71 ± 1.36 (Tab. 3.).
| variables | Ovulation rate at 1st month | T | p | ||
|---|---|---|---|---|---|
| -ve (n = 93) |
+ve (n = 7) |
||||
| Age | Mean ± SD | 31.98 ± 3.54 | 30.29 ± 2.63 | 1.238 | 0.219 |
| Range | 25.0 – 37.0 | 26.0 – 34.0 | |||
| BMI | Mean ± SD | 28.29 ± 2.08 | 27.97 ± 3.0 | 0.383 | 0.702 |
| Range | 25.0 – 32.0 | 25.30 - 31.80 | |||
| AMH | Mean ± SD | 8.22 ± 3.53 | 7.57 ± 2.30 | 0.474 | 0.636 |
| Range | 3.0 – 15.0 | 5.0 – 11.0 | |||
| Endometrial thickness | Mean ± SD | 6.53 ± 0.86 | 6.71 ± 1.36 | 0.352 | 0.736 |
| Range | 5.70-8.90 | 5.70-8.80 | |||
Tab. 3. Correlation between ovulation rate in 1st month and different variables.
t: Student t-test
As regard to ovulation rate in 2nd month and its relation between variables, 10 (10%) pateints ovulated whose:
• Age ranged from 25- 37 years with a mean value 32.30± 3.77 (Tab. 4.).
• BMI ranged from 25.40 - 31.90 kg/m2 with a mean value 28.06 ± 1.93 (Tab. 4.).
• AMH ranged from 3.0 – 15.0 ng/ml with a mean value 8.80 ± 3.97 (Tab. 4.).
• Endometrial thickness ranged from 5.70-8.80 mm with a mean value 6.93 ± 1.34 (Tab. 4.).
• Range 5.70-8.80 5.80-8.90
| Variables | Ovulation rate at 2nd month | t | p | ||
|---|---|---|---|---|---|
| -ve (n =90) |
+ve (n = 10) |
||||
| Age | Mean ± SD | 31.81 ± 3.49 | 32.30 ± 3.77 | 0.417 | 0.677 |
| Range | 25.0 – 37.0 | 25.0 – 37.0 | |||
| BMI | Mean ± SD | 28.29 ± 2.16 | 28.06 ± 1.93 | 0.328 | 0.744 |
| Range | 25.0 – 32.0 | 25.40 - 31.90 | |||
| AMH | Mean ± SD | 8.10 ± 3.41 | 8.80 ± 3.97 | 0.607 | 0.545 |
| Range | 3.0 – 15.0 | 3.0 – 15.0 | |||
| Endometrial thickness | Mean ± SD | 6.50 ± 0.84 | 6.93 ± 1.34 | 0.996 | 0.343 |
| Range | 5.70-8.90 | 5.70-8.80 | |||
Tab. 4. Correlation between ovulation rate in 2nd month and different variables.
t: Student t-test
*: Statistically significant at p ≤ 0.05
As regard to ovulation rate in 3rd month and its relation between variables, 10 (10%) pateints ovulated whose:
• Age ranged from 25- 37 years with a mean value 29.92 ± 3.45 (Tab. 5.)
• BMI ranged from 25.1 - 31.7 kg/m2 with a mean value 28.28 ± 1.99 (Tab. 5.)
• AMH ranged from 3 – 15 ng/ml with a mean value 10.5 ± 3.37 (Tab. 5.).
• Endometrial thickness ranged from 5.8-8.9 mm with a mean value 7.58 ± 1.16 (Tab. 5.).
| Variables | Ovulation rate at 3rd month | t | p | ||
|---|---|---|---|---|---|
| -ve (n =88) |
+ve (n = 12) |
||||
| Age | Mean ± SD | 32.12 ± 3.44 | 29.92 ± 3.45 | 2.085* | 0.040* |
| Range | 25.0 – 37.0 | 25.0 – 37.0 | |||
| BMI | Mean ± SD | 28.27 ± 2.16 | 28.28 ± 1.99 | 0.021 | 0.983 |
| Range | 25.0 – 32.0 | 25.10 - 31.70 | |||
| AMH | Mean ± SD | 7.85 ± 7.85 | 10.50 ± 3.37 | 2.563* | 0.012* |
| Range | 3.0 – 15.0 | 3.0 – 15.0 | |||
| Endometrial thickness | Mean ± SD | 6.40 ± 0.76 | 7.5 8 ± 1.16 | 3.392* | 0.005* |
| Range | 5.70-8.80 | 5.80-8.90 | |||
Tab. 5. Correlation between Ovulation rate in 3rd month and different variables.
t: Student t-test
*: Statistically significant at p ≤ 0.05
As regard to pregnancy rate and its relation between variables, 12 (12%) pateints with positivepregnancy test whose:
• Age ranged from 25 – 36 years with a mean value 29.75 ± 3.7 (Tab. 6.).
• BMI ranged from 25.1 - 31.9 kg/m2 with a mean value 28.97 ± 2.31 (Tab. 6.).
• AMH ranged from 6 – 15 ng/ml with a mean value 11.33 ± 3.03 (Tab. 6.).
• Endometrial thickness ranged from 8.1 – 8.9 mm with a mean value 8.60 ± 0.27 (Tab. 6.).
• Number of follicles ranged from 5-28 foll with mean value 19.25 ± 7.28 (Tab. 6.).
• FSH ranged from 2.57-7.96 IU/mL with mean value 4.43±1.92 (Tab. 6.).
| Variables | Pregnancy rate | t | p | ||
|---|---|---|---|---|---|
| -ve (n = 88) |
+ve (n = 12) |
||||
| Age | Mean ± SD | 32.15 ± 3.39 | 29.75 ± 3.70 | 2.273* | 0.025* |
| Range | 25.0 – 37.0 | 25.0 – 36.0 | |||
| BMI | Mean ± SD | 28.10 ± 2.10 | 28.97 ± 2.31 | 1.206 | 0.231 |
| Range | 25.0-32.0 | 25.10-31.90 | |||
| AMH | Mean ± SD | 7.74 ± 3.29 | 11.33 ± 3.03 | 3.583* | 0.001* |
| Range | 3.0 – 15.0 | 6.0 – 15.0 | |||
| Endometrial thickness | Mean ± SD | 6.26 ± 0.50 | 8.60 ± 0.27 | 15.844* | <0.001* |
| Range | 5.70 – 8.8 | 8.10 – 8.90 | |||
| Number of follicles | Mean ± SD | 15.55 ± 6.77 | 19.25 ± 7.28 | 1.764 | 0.081 |
| Range | 2.0 – 30.0 | 5.0 – 28.0 | |||
| FSH | Mean ± SD | 5.17 ± 1.83 | 4.43 ± 1.92 | 1.307 | 0.194 |
| Range | 2.20-8.11 | 2.57-7.96 | |||
Tab. 6. Correlation between pregnancy rate and different variables.
Number needed to treat
• Number needed to treat = 1/ control event rate – experimental event rate,
• Number needed to treat = 1/ (1-0.71),
• Number needed to treat = 3.44,
• Thus need to treat 4 patients to improve ovulation rate (Tab.7 & 8.).
| Variables | Univariate | #Multivariate | ||
|---|---|---|---|---|
| P | OR (95%C.I) | p | OR (95%C.I) | |
| Age | 0.032* | 0.822 (0.688 – 0.983) | 0.478 | 0.568(0.119-2.708) |
| BMI | 0.232 | 1.192 (0.894 – 1.590) | ||
| AMH | 0.002* | 1.400 (1.128 – 1.739) | 0.691 | 1.279(0.380-4.313) |
| Endometrial thickness | 0.002* | 50.638(3.98-643.6) | 0.040* | 234.5(1.27-4316.4) |
Tab. 7. Univariate and multivariate logistic regression analysis for the parameters affecting pregnancy rate (n = 12 vs. 88).
| Variables | Univariate | #Multivariate | ||
|---|---|---|---|---|
| P | OR (95%C.I) | p | OR (95%C.I) | |
| Age | 0.047* | 0.836(0.701 – 0.997) | 0.399 | 0.887(0.672-1.172) |
| BMI | 0.983 | 1.003(0.755 – 1.332) | ||
| AMH | 0.018* | 1.259(1.041 – 1.523) | 0.764 | 1.047(0.775-1.415) |
| Endometrial thickness | 0.001* | 2.982(1.664-5.342) | 0.002* | 2.743(1.460-5.151) |
Tab. 8. Univariate and multivariate logistic regression analysis for the parameters affecting ovulation rate at 3rd month (n = 12 vs. 88).
Discussion
Polycystic ovary syndrome (PCOS) is considered as one of the most common endocrine disorders in women of reproductive age with a strong genetic component. Infertility or sub fertility is a frequent complaint in women with PCOS that results from anovulatory cycles [15].
Women with PCOS are at increased risk of insulin resistance, inflammation, obesity, type 2 diabetes and cardiovascular diseases, and all of these diseases’ states have been linked with vitamin D insufficiency. Vitamin D insufficiency may contribute to the pathogenesis of PCOS by promoting insulin resistance, which increases the risk of T2DM and cardiovascular diseases [2].
Clomiphene citrate still remains the first line therapy for induction of ovulation in women with PCOS; ovulation can be induced in about 80% of women. Approximately 15% of women with PCOS do not respond to the maximum dose of clomiphene citrate and are considered resistant to this medication; therefore many additive medications can be combined to overcome clomiphene citrate resistance [16].
Diverse mechanisms explain Vitamin D3 role in female reproduction; First, the direct stimulatory effect of vitamin D3 [1, 25(OH) 2D3] on the aromatase gene expression in reproductive tissues. Second, it has been shown that HOXA10 Expression which is essential for endometrial development is upregulated by 1, 25(OH) 2D3 in human endometrial stroma cells. Third, Vitamin D3 and calcium repletion might lead to normalization of menstrual cycles and restoration of ovulation through oocyte activation and maturation [17].
The aim of this study was to assess the effect of oral supplement of vitamin D on patients with clomiphene citrate resistant PCOS.
A clinical trial study was conducted in infertility clinic in Ain Shams Maternity Hospital including130 women who were: PCOS in child bearing age (from 18 to 37 year-old), With body mass index from 25 to 35 & Clomiphene citrate resistant. The duration of the study was 12 months. Patients received clomiphene citrate with vitamin D for 3 cycles.
• The age of the patients ranged from 25-37 years with a mean value 31.86 ± 3.5 years. The period of infertility ranged from 2-7 years with a mean value 4.78 ± 1.16 years. The BMI of the patients ranged from 25-35 kg/m2 with a mean value 28.27 ± 2.13 kg/m2.
• After administration of vit D, 29 (29%) patients ovulated.
• As regard to ovulation rate each month, the 3rd month showed higher ovulation rate 12 (12%) than 1st and 2nd month 7(7%) & 10 (10%), respectively.
• As regard to pregnancy rate, 12 (12%) patients showed positive pregnancy tests where 5% showed clinical pregnancy and 88 (88%) patients were negative.
• As pregnancy rate each month, 3rd month showed higher pregnancy rate (7% chemical pregnancy & 3% clinical pregnancy) than 1st month (2% chemical & 1% clinical) and 2nd month ( 3% chemical &1% clinical).
Agreement with the current study, Yahya’s, et al. [8] found that vitamin D3 supplementation for 2 months in clomiphene citrate resistant PCOS patients produce 18/24 (75%) positive ovulation and 3/24 pregnancies (12.5%) the study enrolled 41 clomiphene citrate resistant PCOS patients whose age ranged from (18-34). They were divided into two groups, group 1: included hypovitaminosis D PCOS patient received clomiphene citrate 100mg daily (for 5 days in each induction month) plus vitamin D 10000IU daily (2 months), and group 2: included PCOS patient with insufficient vitamin D status received clomiphene citrate 100mg daily (for 5 days in each induction month) plus Co enzyme Q10 200 mg daily as replacement therapy (2 months). Baseline and after 2 months fasting blood samples used to measure hormonal and oxidative stress biomarkers, transvaginal ultrasound for determination of ovulation, and pregnancy test was done for those patient who have no menstruation for two weeks after HCG injection. The study revealed that improvement in ovulation outcome and 4/21 PCOS infertile patients became pregnant after discontinuation of vitamin D3 treatment, and this pregnancy occurred within the first month after correction of their endogenous vitamin D status in this study. Also supplementation with vitamin D3 resulted in significant decrease in free testosterone level (P=0.012), LH (p=0.021) and LH: FSH ratio (P=0.019). The overall ovulation was in 75% of PCO pateints mean while overall pregnancy was 15%. Thus supplementations with vitamin D3 to clomiphene citrate resistance PCOS pateints resulted in improving hormonal profile and ovulation outcome.
Also Radwa, et al. studied the efficacy of vitamin D combined with clomiphene citrate in ovulation induction in overweight women with PCOS. The results showed that 86 (92.5%) of women received Vitamin D had successful ovulation after the induction cycles compared with 73 (78.5%) in the placebo group (p = 0.007). the absolute and relative risk reduction was 14% and 65% respectivily. biochemical and clinical pregnancy occurred in 61.3 and 50.5% in the treatment group and in 49.5 and 39.8% in the control group. The study enrolled 186 eligeible women underwent ovulation induction by clomiphene citrate (Clomid, Aventis) 50 mg tablet twicw daily starting from day 3 menses for 5 days combined with either oral vitamin D (Ossofortin, EVA PHARMA) 10000 IU twice weekly and calcium (Calciprex, Marcyl pharmaceutical) 1250 twice daily or to receive placebo with Calcuim fro three successive induction cycles
Th vitamin D or placebo supplementation started 1 month before induction cycles. The mean diameter of the dominant follicle was 18.07 ± 1.37 mm in the treatment group and was 16.79 ± 3.00 mm with p < 0.001 in the placebo group. Thus vitamin D group showed an increase in both the clinical and biochemical pregnancy rates compared to placebo. Thus in subfertile PCOS women undergoing ovulation induction vitamin D supplementation significantly improved the ovulation rate [18].
The results were in agreement with the study of Rashidi, et al. enrolled 60 infertile PCOS patients in randomized control trail, divided into 3 groups: Group 1: recieved 1000 mg of calcium plus 400 IU of Vitamin D per day orally. Group 2: received the same plus 1500 mg/day metformin. Group3: recieved 1500 metformin only. The pateints were treated for 3 months and followed up a further 3 months .Regularity of menses, number of large follicles (>14 mm) and pregnancy rates were compared among the three groups. He found that higher number of dominant follicles in patients receive calcium-vitamin D plus metformin comparing to only calcium-vitamin D or only metformin during the 2-3 months of follow up (P=0.03) [19].
Agreeing also with current study, Zhuang, et al. aimed to explore the clinical efficacy of vitamin D combined with metformin and clomiphene in treating infertile poly cystic ovary syndrome pateints. The study showed that ovulation rate in study group 188 (92.1%) is significatly higher than that of the control group 106 (55.2%). Pregnancy rate in study group 136 (66.6%) and control group 89 (46.3%). The study enrolled 396 infertile poly cystic ovarian syndrome cases, among them 204 cases treated with vitamin D combined with metformin and clomiphene were set as the study group; 192 cases treated only with metformin and clomiphene were set as the control group (p<0.05). The ovarian volume and endometrial thickness were recorded before and after treatment. Results showed that there was significant improvement in clinical symptoms and endocrine conditions; also there was agreat enhancement in ovulation and pregnancy rate [20].
Also study of Gupta, et al. enrolled fifty PCOS women who were randomized then were given vitamin D or placebo once weekly for 12 weeks. The study concluded that there was a benificial effect of vitamin D supplementation on ovulatory dysfunction and blood pressure .In post supplemetation, there were decrease in insulin resistance and increase insulin sensitivity. In the study decreased serum fasting insulin level and fasting blood sugar after vitamin D supplementation suggest underlying role of vitamin D in glucose hemostasis [21].
Also Tehrani, et al. found that dominant follicles were higher in metformin plus calcium and Vitamin, by studying the effect of calcium and vitamin D supplementation on menstrual cycle, body mass index and hyperandrogenism state of women with PCOS. After trial, the frequency of hirsutium and acne were not different among groups, but frequency of regular menses and dominant follicle were significally higher in group that received metformin and metformin plus calcium with vit D compared those who received calcim with vitamin D only and placebo [22].
However, other studies have mostly yielded mixed results (Pergialiotis et al., 2017), aimed to investigate the impact of vitamin D on hormonal and metabolic profile of PCOS women. Nine studies were included in this meta-analysis which investigated the impact of vitamin D supplementation in 647 patients. According to meta-analysis neither serum testosterone (MD 0.04 ng/mL, 95% CI −0.09 to 0.17) nor serum LH (MD −0.48 IU/mL, 95% CI −1.97 to 1.00) were significantly affected by vitamin D supplementation in any of the subgroup comparisons. On the contrary, serum DHEAS was significantly affected by vitamin D (MD −32.24 μg/dL, 95% CI −32.24 to −14.01) an effect which was mainly affected by the vitamin D vs placebo comparison. Vitamin D supplementation did not have an impact on fasting glucose (MD 0.42 mg/dL, 95% CI −2.75 to 3.60) or fasting insulin (MD 1.27 μU/mL, 95% CI −1.42 to 3.97) levels. HOMA-IR was, however, increased among patients that received placebo compared to vitamin D (MD 0.52, 95% CI 0.39-0.65). There is no evidence to support that vitamin D supplementation significantly benefits PCOS patients [23].
Also disagreeing with the current study Abdel Moneam study showed that if vitamin D is given with clomiphene citrate and HMG, there was no statistically significant improvement in the follicular growth pattern or leptin level. The study was enrolled 40 female candidates with polycystic ovary syndrome They are divided into two groups Study group 20 patients received vitamin D, clomiphene and HMG. Control group 20 patients received only clomiphene and HMG. All women are subjected to measurement of leptin and follicular growth before and after treatment. 25 hydroxyvitamin D3 was done in the study group before and after treatment and in control group only before treatment .There was no significant improvement in the follicular growth in the group treated with VIT D in comparison to the other group (65% vs 55 %) respectively with p value =0.519 [24].
Meanwhile, Dravecká, et al. investigate prevelance of vitamin D deficiency and its relation to clinical and biochemical findings in PCOS and controls.the study were done on 99 PCOS women and 66 controls. He reported that there was no significant difference in 25 OH D levels between PCOS women and controls. Thus insulin resistance and other metabolic abnormalities in PCOS women seem to be related to PCOS rather than vitamin D deficiency. He found no significant change in parameters (FT, DHEAS, LH, LH/FSH ratio) after 6 months therapy in all study groups [25].
Unlike the current study, David, et al. studied the effect of metformin on ovulation and pregnancy rate in Clomiphene citrate resistant poly cystic ovarian syndrome women. Ovulation rate was 75% (9 of 12 participant) and pregnancy rate was 55% (6 of 11 participants) While the current study showed 29 (29%) out of 100 pateints ovulated and 12 (12%) got pregnant by giving vitamin D. David, et al. study was a randomized controlled double blinded placebo controlled trial. Participants received placebo or metformin, 500 mg three times daily for 7 weeks .Then metformin or placebo was continued with clomiphene citrate (50 mg for 5 days). Pateints completed the study when they had had 6 ovulatory cycles. Comparisons between the groups were significant. Results showed that metformin and placebo groups, 9 of 12 participants (75%) and 4 of 15 participants (27%) ovulated, and 6 of 11 participants (55%) and 1 of 14 participants (7%) conceived, respectively [26]. Elnashar studied the effect of dexamethasone on clomiphene citrate resistant poly cystic ovarian syndrome women. He found that three fourths of women given dexamethasone and 15% of placebo recipients ovulated, and 40% and 5%, respectively, became pregnant. While the current study showed 29 (29%) out of 100 pateints ovulated and 12 (12%) got pregnant by giving vitamin D .The investigators concluded that a significant number of Clomiphene citrate resistant infertile women with PCOS will benefit from adding a brief, high-dose course of Dexamethasone. Clomiphene citrate was given in a dose of 100 mg daily from cycle day 3 to day 7. In addition, patients received either 2 mg daily of deamethasone orally from day 3 to day 12 of the cycle or placebo tablets. Ultrasonography was used to detect ovarian follicles more than 18 mm in size. HCG was given and women were advised to have timed intercourse. Steroid-treated women had more large follicles and significantly greater endometrial thickness than did placebo recipients. They also had significantly higher rates of ovulation and pregnancy [7].
Also Mahmoud and Abdel Razik, studied the effect of adding prednisolone during Ovulation Induction with Clomiphene Citrate in Lean Women with Clomiphene Citrate Resistant Polycystic Ovarian Syndrome. Results showed that there were significantly higher rates of ovulation (58.7% versus 23%) in the prednisolone group. The clinical pregnancy rate was significantly higher in the prednisolone group. While the current study showed 29 (29%) out of 100 pateints ovulated and 12 (12%) got pregnant by giving vitamin D The study enrolled 300 infertile lean women with clomiphene citrate (CC) resistant PCOS were randomly divided into two groups. Group 1:150 patient received clomiphene citrate (5 consecutive days of 150mg daily starting from the second day of the cycle) and prednisolone tablet (10 consecutive days of 10mg daily starting from the second day of the cycle). Group 2:150 patient received the same protocol of CC plus placebo [27].
Asadi, et al. 2014 found that endometrial thickness was significantly different in PCOS women treated with vitamin D versus the placebo group (p=0.003). The study was done on 110 infertile PCOS pateints underwent IUI divided into 2 groups: one received vit D and other received placebo [28].
Conclusion
Supplementation with oral vitamin D improved fertility outcome in clomiphene citrate resistance PCOS patients.
Authors Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- A pilot study: effects of decreasing serum insulin with diazoxide on vitamin D levels in obese women with polycystic ovary syndrome. Trans Am Clin Climatol Assoc. 2012;123:209.
- Yildizhan R, Kurdoglu M, Adali E, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280(4):559-63.
- Teegarden D, Donkin SS. Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev. 2009;22(1):82-92.
- Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102(2):460-8.
- Wong HY, Li HW, Lam KS, et al. Independent association of serum vitamin D with anti‐Mullerian hormone levels in women with polycystic ovary syndrome. Clin Endocrinol. 2018;89(5):634-41.
- Butts SF, Seifer DB, Koelper N, et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J Clin Endocrinol Metab. 2019;104(2):369-78.
- Elnashar A, Abdelmageed E, Fayed M, et al. Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod. 2006;21(7):1805-8.
- Yahya AA, Abdulridha MK, Al-Rubuyae BJ, et al. The effect of vitamin D and co-enzyme Q10 replacement therapy on hormonal profile and ovulation statusin women with clomiphene citrate resistant polycystic ovary syndrome. J Pharm Sci Res. 2019;11(1):208-15.
- Azziz R. Diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. The J Clin Endocrinol Metab. 2006;91(3):781-5.
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:433-53.
- Maktabi M, Chamani M, Asemi Z. The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res. 2017;49(07):493-8.
- Dos Santos E, Dieudonné MN, Leneveu MC, et al. In vitro effects of chorionic gonadotropin hormone on human adipose development. J Endocrinol. 2007;194(2):313-26.
- Scaramuzzi RJ, Radford HM. Factors regulating ovulation rate in the ewe. Reproduction. 1983;69(1):353-67.
- Annan JJ, Gudi A, Bhide P, et al. Biochemical pregnancy during assisted conception: a little bit pregnant. J Clin Med Res. 2013;5(4):269.
- Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25.
- Jamilian M, Foroozanfard F, Rahmani E, et al. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients. 2017;9(12):1280.
- Özer A, Bakacak M, Kıran H, et al. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekoloiapolska. 2016;87(11):733-8.
- Rasheedy R, Sammour H, Elkholy A, et al. The efficacy of vitamin D combined with clomiphene citrate in ovulation induction in overweight women with polycystic ovary syndrome: a double blind, randomized clinical trial. Endocrine. 2020;69(2):393-401.
- Rashidi B, Haghollahi F, Shariat M, et al. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol. 2009;48(2):142-7.
- Zhuang L, Wei CU, Jianxiang CO, et al. Efficacy of vitamin D combined with metformin and clomiphene in the treatment of patients with polycystic ovary syndrome combined with infertility. Iran J Public Health. 2019;48(10):1802.
- Gupta T, Rawat M, Gupta N, et al. Study of effect of vitamin D supplementation on the clinical, hormonal and metabolic profile of the PCOS women. J Obstet Gynecol India. 2017;67(5):349-55.
- Tehrani HG, Mostajeran F, Shahsavari S. The effect of calcium and vitamin D supplementation on menstrual cycle, body mass index and hyperandrogenism state of women with poly cystic ovarian syndrome. J Res Med Sci. 2014;19(9):875.
- Pergialiotis V, Karampetsou N, Panagopoulos P, et al. The effect of Vitamin D supplementation on hormonal and glycaemic profile of patients with PCOS: A meta‐analysis of randomised trials. Int J Clin Pract. 2017;71(6):e12957.
- Moneam AA. The effect Of Vitamin D Replacement Therapy On Serum Leptin And Follicular Growth Pattern In Women With Resistant Polycystic Ovary. Master Thesis, Departments of obstetrics and gynecology, and clinical pathology, Faculty of Medicine, Fayoum University. 2014.
- Dravecká I, Figurova J, Javorský M, et al. The effect of alfacalcidiol and metformin on phenotype manifestations in women with polycystic ovary syndrome-a preliminary study. Physiological research. 2016;65(5):815.
- Vandermolen DT, Ratts VS, Evans WS, et al. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril. 2001;75(2):310-5.
- Thabet M, Abdelrazik MM. Adding Prednisolone During Ovulation Induction with Clomiphene Citrate in Lean Women with Clomiphene Citrate Resistant Polycystic Ovarian Syndrome. The Egypt J Infertil Steril. 2019;23(1):16-22.
- Asadi M, Matin N, Frootan M, et al. Vitamin D improves endometrial thickness in PCOS women who need intrauterine insemination: a randomized double-blind placebo-controlled trial. Arch Gynecol Obstet. 2014;289(4):865-70.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Al Hassan Mohammad Khedr, Rana Alaa Eldin*, Hesham Harb and Laila Aly FaridCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.