Research - (2022) Volume 0, Issue 0
Effect of subcutaneous wound infiltration of different drugs in patients with Cesarean section: A prospective cohort study
Amr Sobhy1,2*, Shaimaa W. Zeinah3, Waleed H. Alkhamis4, Suaad Ali AlEdrisy5, Mai A. Ebeid6 and Mohamed I. Taema6,7Received: 03-Mar-2022, Manuscript No. gpmp-24-150015; Editor assigned: 05-Mar-2022, Pre QC No. P-150015; Reviewed: 21-Mar-2022, QC No. Q-150015; Revised: 26-Mar-2022, Manuscript No. R-150015; Published: 31-Mar-2022
Abstract
Background: The requirement for postoperative analgesia is increasingly necessary as the rate of cesarean section (CS) is rapidly rising. Aim: to assess the impact of administering tramadol via injection at the incision site before closing the skin in patients having a cesarean section on postoperative pain and the requirement for pain-relieving medication.
Methods: This Prospective cohort study was conducted at El Madinah National Hospital in El Madinah al Munawara, Saudi Arabia, from June 2021 to December 2021. Forty-eight women who were undergoing cesarean section delivery were allocated into two groups, with each group assigned to receive either tramadol or lidocaine subcutaneously before the closure of the skin in the cesarean section. The pain was evaluated at 6-, 12, and 24-hours post-operation using the visual analog scale (VAS).
Results: The tramadol group showed significantly lower pain levels (VAS scores) compared to the lidocaine group at 6 hours (p<0.001) and 12 hours (p<0.01). At 24 hours, the VAS score was significantly similar to the Lidocaine group. The time to first analgesic demand was significantly longer in the Tramadol group: 5.78±2.84 hours in the Lidocaine group, with a p-value of <0.001.
Conclusion: Subcutaneously injecting Tramadol was more effective in reducing pain scores compared to lidocaine.
Keywords
Tramadol; Pain score; Lidocaine; Cesarean section
Introduction
Cesarean Section (CS) is considered a major operation and is commonly performed in modern times. While CS offers certain advantages, such as reducing the likelihood of birth injuries (e.g., asphyxia, shoulder dystocia, and fractures), it can also result in significant postoperative discomfort [1,2].
Effective management of pain following a Cesarean section can contribute to a quicker recovery, shorter hospital stay, and early bonding between mother and newborn. Inadequate pain relief can lead to prolonged hospitalization and additional health issues [3].
"Local infiltration analgesia" refers to using a "large amount of diluted, long-lasting local anesthetic" in tissue structures to relieve pain. Local anesthetic wound infiltration is primarily used for minor surgeries such as laceration repair, skin surgery, and treatment of painful oral or genital lesions. It can also be used as a supplement to general anesthesia for various surgical procedures. Certainly! Here is the reworded text:
Various research studies utilized lidocaine 2%, resulting in decreased postoperative pain and reduced need for additional pain medication. In a separate study, 1% of lidocaine was administered, but there was no significant difference in postoperative pain levels or requests for pain relief between the group receiving lidocaine and the group receiving a placebo [4-6].
Another study showed significantly better analgesia and morphine-sparing effect when administering subcutaneous pethidine or tramadol after cesarean in comparison to long-acting bupivacaine [7,8].
This study aimed to assess the impact of administering tramadol via injection at the incision site prior to closing the skin in patients having a cesarean section on postoperative pain and the requirement for pain-relieving medication.
Materials and Methods
This Prospective cohort study was conducted at El Madinah National Hospital in El Madinah al Munawara, Saudi Arabia, from June 2021 to December 2021. Forty-eight women who were undergoing cesarean section delivery were allocated into two groups, with each group assigned to receive either tramadol or lidocaine subcutaneously before the skin in the cesarean section closed. Our hospital ethically approved the study, and all women gave informed consent in line with the Declaration of Helsinki.
Inclusion criteria: Patients admitted for elective Repeated Cesarean Section and willing to participate in the study.
Exclusion criteria: Women who had an emergency CS or had a significant medical issue, a bleeding disorder, a drug addiction, or an allergy to the medication used in the study were not included.
Procedure The 48 participants who were undergoing cesarean section delivery were separated into two cohorts to be administered tramadol (group A) and lidocaine (group B) subcutaneously before the closure of the skin during the procedure. All the patients underwent a standard cesarean section under spinal anesthesia. At the time of skin closure, the incision was infiltrated by 20 ml of 1% lidocaine hydrochloride in group B (n=24), or 50 mg tramadol hydrochloride (Tramadol 50mg/ml Solution for Injection or Infusion, Beacon Pharmaceuticals Ltd. UK) diluted in 20 ml of 0.9 saline in group A (n=24). VAS is widely recognized as the primary tool for pain assessment in research. It involves a 100mm horizontal line with two endpoints, one indicating no pain and the other indicating the most severe pain. Patients were instructed to mark the pain level they were experiencing on the line. Patients were given Diclofenac sodium 75mg (Voltaren, Novartis—Switzerland) through intramuscular injection as needed for postoperative pain relief.
The primary outcome was the VAS score, measured 6, 12, and 24 hours after the operation.
The secondary outcomes included the duration until the first request for pain relief and the total amount of diclofenac consumed within 24 hours in the two experimental groups.
Sample size justification
Before the study, the number of patients required in each group was determined after a power calculation according to the data obtained [8]. In the study, VAS/24th hrs. in the local anesthetic Group was 2.12 ± 0.99 compared to the Tramadol Group, which was 1.140 ± 0.88; based on this assumption through this previous study, the effect size was (f=1.07). A sample size of 24 patients in each group was determined to provide 95% power for independent samples T-test at the level of 5% significance and Confidence interval 95% using G. Power 3.19.2 software.
Statistical analysis
The data is displayed as mean, Standard Deviation (SD), median, and range values. When comparing more than two means for parametric data, an Independent-samples t-test of significance was used when comparing between two means, and the Chi-square (x2) test of significance was employed. The significance level is set at P ≤ 0.05. The statistical analysis was conducted using IBM SPSS Statistics for Windows, Version 23.0, by IBM Corp. located in Armonk, NY.
Results
The Lidocaine and Tramadol groups had similar general characteristics, obstetric history, and past medical and surgical history among the included women. Tab. 1. demonstrates no statistically significant variances in age, body mass index (BMI), and gestational age at delivery among the studied groups.
| Patients’ Characteristics | Lidocaine Group (n=24) | Tramadol Group (n=24) | Test value | p-value | Sig. |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 30.45 ± 4.06 | 30.29 ± 4.15 | 0.130 | 0.897 | NS |
| Range | 24-37 | 25-37 | |||
| BMI (kg/m2) | |||||
| Mean ± SD | 27.18 ± 3.50 | 28.47 ± 3.27 | -1.317 | 0.194 | NS |
| Range | 21-33 | 24-33 | |||
| Gestational age (weeks) | |||||
| Mean ± SD | 39.79 ± 1.56 | 39.66 ± 1.65 | 0.288 | 0.775 | NS |
| Range | 37-42 | 38-42 | |||
p-value >0.05 is insignificant
Tab. 1. Comparison between lidocaine group and tramadol group as regards patients’ characteristics.
In the Lidocaine and Tramadol group, there were five women (20.8%) and four women (16.7%), respectively, who were primigravid (p=0.719). Among women in the Lidocaine and Tramadol group, 19 women (79.2%) and 20 women (83.3%) had a history of one or more previous cesarean sections (p=0.719). The Tramadol group's VAS scores were significantly lower than the Lidocaine group at six h and 12 h. At 24 h, (p<0.01) (Tab. 2. and Fig. 1.).
| Pain scores | Lidocaine Group (n=24) | Tramadol Group (n=24) | Test value | p-value | Sig. |
|---|---|---|---|---|---|
| VAS at 6h | |||||
| Mean ± SD | 3.50 ± 0.88 | 2.29 ± 0.44 | 3.522 | 0.001 | HS |
| Range | 2-5 | 1-4 | |||
| VAS at 12h | |||||
| Mean ± SD | 4.96 ± 0.98 | 3.58 ± 0.85 | 3.069 | 0.004 | S |
| Range | 3-7 | 1-6 | |||
| VAS at 24h | |||||
| Mean ± SD | 3.88 ± 0.92 | 2.88 ± 0.70 | 2.515 | 0.015 | S |
| Range | 2-5 | 1-5 | |||
Using: t-Independent Sample t-test for Mean ± SD;
p-value >0.05 is insignificant; *p-value <0.05 is significant; **p-value <0.001 is highly significant
Tab. 2. Comparison between lidocaine group and tramadol group as regards pain score.
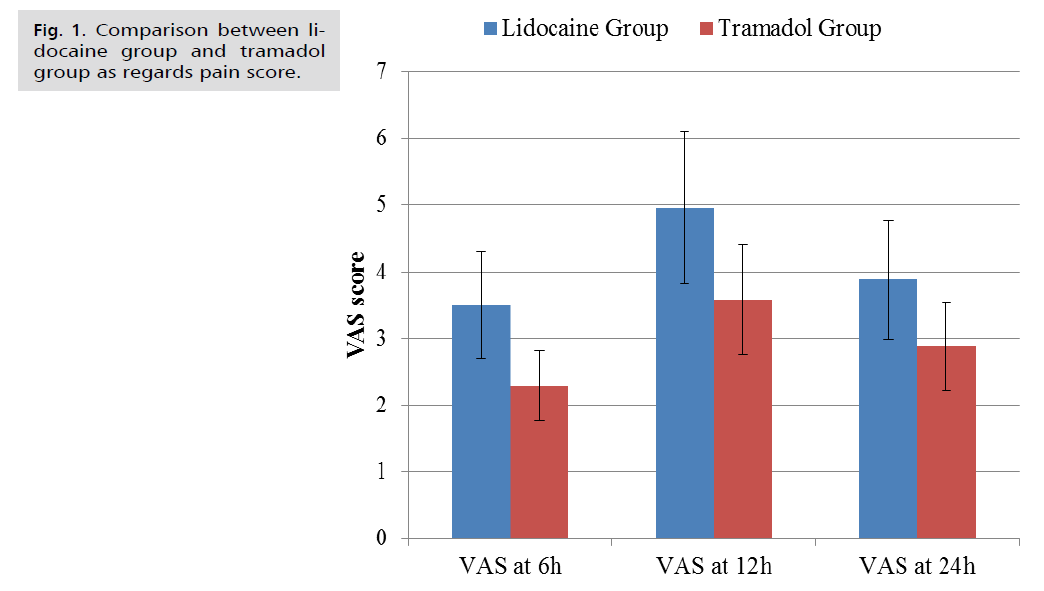
Fig 1. Comparison between lidocaine group and tramadol group as regards pain score.
The Tramadol group had a significantly longer time to first analgesic request compared to the Lidocaine group, with p-value (p=0.001), as shown in Tab. 3. and Fig. 2.
| Time to first analgesic “hrs.” | Lidocaine Group (n=24) | Tramadol Group (n=24) | Test value | p-value | Sig. |
|---|---|---|---|---|---|
| Mean ± SD | 2.67 ± 0.61 | 6.17 ± 1.48 | -6.295 | 0.001 | HS |
| Range | 1-4 | 2-10 |
Using: t-Independent Sample t-test for Mean ± SD;
**p-value <0.001 is highly significant
Tab. 3. Comparison between lidocaine group and tramadol group as regard time to first analgesic.

Fig 2. Mean time to first analgesic request in the three study groups.
In terms of postoperative analgesic consumption, significantly fewer patients in the Tramadol group required diclofenac at 24 hours compared to the Lidocaine groups. The number of women requiring analgesia at 6 hours and 12 hours was similar across all two study groups with no statistically significant difference. There was a highly statistically significant difference in the lower number of patients in the tramadol group (Tab. 4. and Fig. 3.).
| Diclofenac consumption | Lidocaine Group (n=24) | Tramadol Group (n=24) | Test value | p-value | Sig. |
|---|---|---|---|---|---|
| At 6 h | 3 (12.5%) | 5 (20.8%) | 0.600 | 0.439 | NS |
| At 12 h | 23 (95.8%) | 20 (83.3%) | 2.009 | 0.156 | NS |
| At 24 h | 22 (91.7%) | 13 (54.2%) | 8.545 | 0.003 | S |
p-value >0.05 is insignificant; *p-value <0.05 is significant; **p-value <0.001 is highly significant
Tab. 4. Comparison between lidocaine group and tramadol group as regard analgesic consumption.
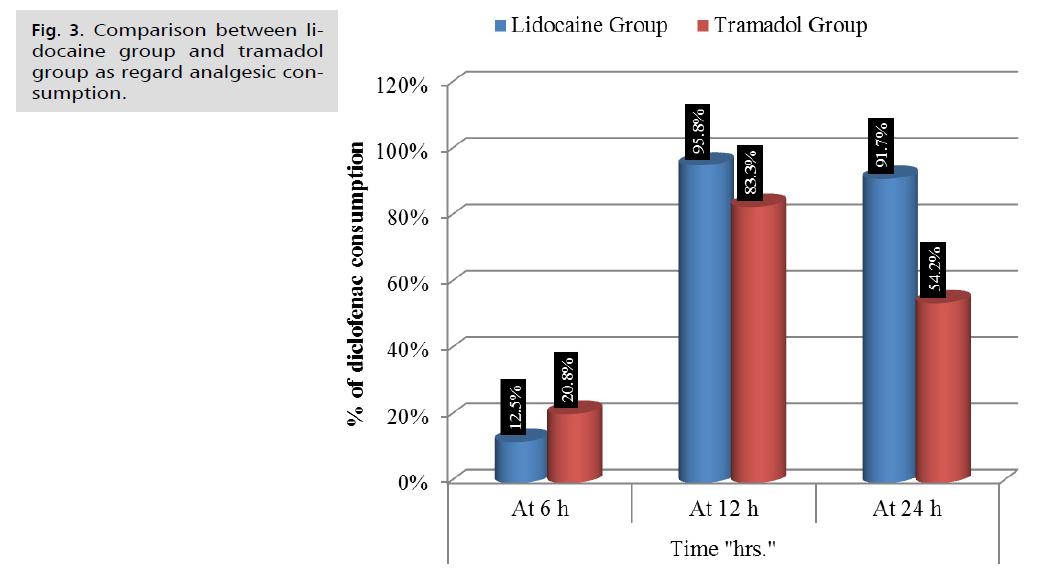
Fig 3. Comparison between lidocaine group and tramadol group as regard analgesic consumption.
As indicated in Tab. 5. and Fig. 4. Additionally, the cumulative 24-hour consumption of diclofenac was significantly lower in the Tramadol group compared to the Lidocaine group, (p=0.001).
| Cumulative 24-hour diclofenac consumption | Lidocaine Group (n=24) | Tramadol Group (n=24) | Test value | p-value | Sig. |
|---|---|---|---|---|---|
| Single dose | 0(0.0%) | 5(20.8%) | 30.496 | 0.001 | HS |
| Three dose | 21(87.5%) | 2(8.3%) | |||
| Two dose | 3(12.5%) | 17(70.8%) |
**p-value <0.001 is highly significant
Tab. 5. Comparison between lidocaine Group and Tramadol Group according to Cumulative 24-hour diclofenac consumption.
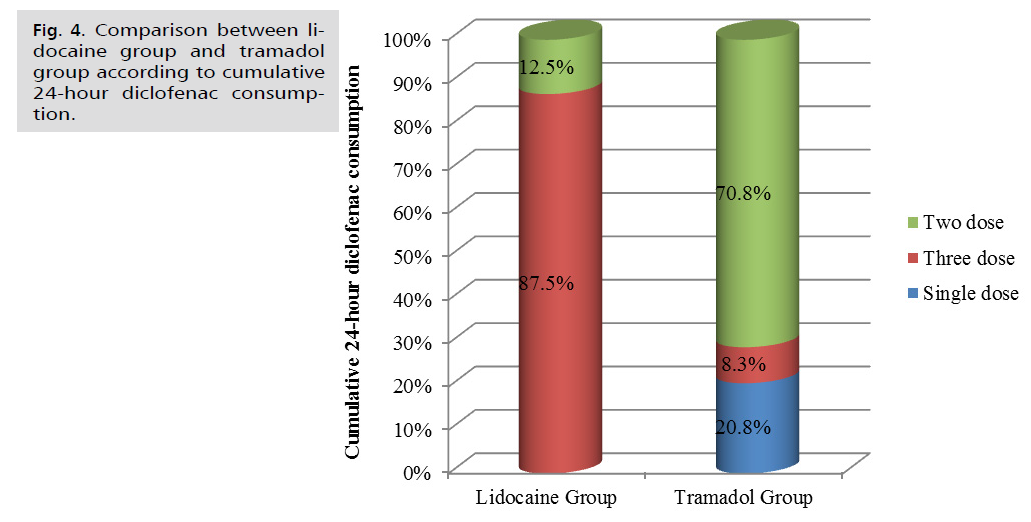
Fig 4. Comparison between lidocaine group and tramadol group according to cumulative 24-hour diclofenac consumption.
Discussion
Our results interpretation and their comparison to other studies
Our study demonstrated that subcutaneous infiltration of tramadol led to a significant reduction in VAS scores compared to lidocaine at 6, 12, and 24 hours. Additionally, tramadol resulted in a significantly longer time to first analgesic request and a notable decrease in total analgesic consumption over 24 hours. The time to first analgesic request and the total analgesic consumption over 24 hours was similar between lidocaine and placebo. These findings contrast with the results of Ghenaee, et al., who conducted a study involving 100 cases randomized to receive lidocaine 2% (4 mg/kg diluted in 30 mL of normal saline). They concluded that the injection of lidocaine 2% into the wound of a cesarean section incision reduced postoperative pain and decreased the need for additional analgesia [4]
The findings we obtained were corroborated by Kessous, et al. in their Randomized Controlled Trial (RCT), which examined the use of a 1% lidocaine solution injected into the incision site during cesarean deliveries. They concluded that there was no notable disparity in postoperative pain scores or analgesic requests between the lidocaine and placebo groups [5].
The evidence is backed up by a Randomized Controlled Trial (RCT) examining the pain-relieving effects of tramadol vs. saline injection under the skin for lower abdominal surgeries. The study found that tramadol led to a significant reduction in pain and opioid usage [6].
In our research, tramadol wound infiltration proved more effective than lidocaine wound infiltration, as evidenced by Jabalameli, et al.'s study. The study compared the effects of pethidine, tramadol, bupivacaine, and placebo on 120 patients undergoing cesarean section, They randomized 120 patients into four groups f. Group P received Pethidine, Group T received Tramadol, Group B received Bupivacaine, and Group C was the control group. Pain intensity and opioid consumption were evaluated at various time points after surgery. The VAS scores were significantly lower in groups T and P compared with groups B and C, except for 24 hours (VAS rest) and 6 hours (VAS on coughing) postoperatively. The number of patients requiring morphine was significantly different between the groups, except for 2 and 6 hours postoperatively [7]. These results are in line with our study, which found that pethidine and tramadol were more effective than the other groups in reducing postoperative pain. Additionally, the pethidine and tramadol groups required significantly less additional analgesia. These findings may be attributed to the prolonged action of tramadol.
In a study by Sachidananda, et al., tramadol enhanced the effects of bupivacaine, resulting in an extended pain-free period and reduced the need for additional analgesia [8].
The findings of the present research contradicted those of Jayashree, et al. Their investigation involved 60 women who underwent cesarean sections under spinal anesthesia. They compared tramadol to bupivacaine and discovered that bupivacaine exhibited superior analgesic effects compared to tramadol. Their study revealed that tramadol had a significant pain-relieving effect and prolonged duration [9].
Clinical implication of our study
The data indicated a high frequency of postoperative pain and its significant impact on the mother, family, medical professionals, and healthcare services [10]. Our findings suggest that local administration of tramadol in the surgical wound led to notably lower pain levels, longer duration before the first request for pain relief, and reduced overall consumption of pain medications within 24 hours. Tramadol wound infiltration is a favorable option for managing postoperative pain after a cesarean section.
Limitations and Strengths of the Study
The primary limitation of this study is the insufficient number of patients, as the participation of Arab women is restricted due to their conservative Islamic culture. Additionally, there was a lack of randomization in this study because private hospitals lack RCT units. Private patients were resistant to the concept of randomization and preferred to be offered the best option.
Recommendation for Future Research
Additional research is required to assess the impact of bupivacaine and other opioid injections in cesarean section incisions.
Conclusion
The administration of tramadol via local wound injection led to lower pain scores, a longer duration until the first request for pain relief, and a reduced overall consumption of analgesic medication over 24 hours. Tramadol wound injection is a favorable option for post-operative pain management in the case of cesarean sections.
References
- Roofthooft E, Joshi GP, Rawal N, et al. PROSPECT guideline for elective caesarean section: Updated systematic review and procedure‐specific postoperative pain management recommendations. Anaesth. 2021;76(5):665-680.
- Gamez BH, Habib AS. Predicting severity of acute pain after cesarean delivery: A narrative review. Anesth Analg. 2018;126(5):1606-1614.
- Paladini G, Di Carlo S, Musella G, et al. Continuous wound infiltration of local anesthetics in postoperative pain management: Safety, efficacy and current perspectives. J Pain Res. 2020:285-294.
- Ghenaee MM, Rahmani S, Jafarabadi MI. Local lidocaine 2% in postoperative pain management in cesarean delivery. J Family Reprod Health. 2015;9(1):19.
- Kessous R, Wiznitzer A, Polachek H, et al. Preoperative analgesia with local lidocaine infiltration for post cesarean delivery pain management. J Matern Fetal Neonatal Med. 2012;25(7):1131-1134.
- Jabalameli M, Hazegh P, Talakoub R. Preemptive subcutaneous tramadol for post-operative pain in lower abdomen surgeries: A randomized double blinded placebo-control study. Adv Biomed Res. 2013;2(1):68.
- Jabalameli M, Safavi M, Honarmand A, et al. The comparison of intraincisional injection tramadol, pethidine and bupivacaine on postcesarean section pain relief under spinal anesthesia. Adv Biomed Res. 2012;1(1):53.
- Sachidananda R, Joshi V, Shaikh SI, et al. Comparison of analgesic efficacy of wound infiltration with bupivacaine versus mixture of bupivacaine and tramadol for postoperative pain relief in caesarean section under spinal anaesthesia: A double-blind randomized trial. J Obstet Anaesth Crit Care. 2017;7(2):85-89.
- Jayashree V, Latha K, Dhakshinamoorthy M, et al. Intraincisional injection of tramadol vs. bupivacaine in post-caesarean pain relief. Int J Clin Obstet. 2019;3:355-360.
- Yimer H, Woldie H. Incidence and associated factors of chronic pain after caesarean section: A systematic review. J Obstet Gynaecol Can. 2019;41(6):840-854.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Amr Sobhy1,2*, Shaimaa W. Zeinah3, Waleed H. Alkhamis4, Suaad Ali AlEdrisy5, Mai A. Ebeid6 and Mohamed I. Taema6,72Department of Obstetrics and Gynecology, El Madinah National Hospital, El Madinah al Munawara, Saudi Arabia
3Department of Anaesthesia, Ain Shams University, Cairo, Egypt
4Department of Obstetrics and Gynecology, College of Medicine, King Saud University, King Saud University Medical City, Riyad City, Saudi Arabia
5Department of Obstetrics and Gynecology, National Guard Health Affairs, Women’s Health Hospital, Ministry of the National Guard, Riyadh City, Saudi Arabia
6Department of Clinical Pharmacology, Faculty of Medicine, Ain Shams University, Egypt
7Department of Obstetrics and Gynecology, United doctors hospital, Jeddah, Saudi Arabia
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.