Research - (2025) Volume 20, Issue 2
Effect of intraoperative glove changes on post-cesarean infection rates: A systematic review and meta-analysis
Salwa Neyazi1, Khalid Akkour1, Nada Alayed1, Omar Zidan1, Eman Al Shehri1, Shadan Binsaeedan1, Mashael Alshebly1, Mustafa Smisim1, Ahmed Sherif1,2, Alhassan Khedr3*, Sondos Al Hawamdeh4, Mohammed A. Alatawi5,6, Mohammad Atlam, Amal Kalifa7 and Karim Abdelsalam8Received: 01-Jun-2025, Manuscript No. gpmp-25-167457; Editor assigned: 03-Jun-2025, Pre QC No. P-167457; Reviewed: 16-Jun-2025, QC No. Q-167457; Revised: 23-Jun-2025 Published: 30-Jun-2025
Abstract
Background/Aim: Infections following Cesarean Sections (CS) are a notable risk. This systematic review investigates the effectiveness of changing gloves during surgery in preventing wound infections, endometritis, and febrile morbidity, focusing on whether changes occur before or after placental delivery. Methodology: Adhering to PRISMA guidelines (PROSPERO CRD420251074484), a total of eight Randomized Controlled Trials (RCTs) involving 2,168 women were sourced from databases such as MEDLINE, Scopus, and Cochrane Central, along with manual searches. The studies included were those comparing glove changes with standard care during CS. The Cochrane RoB 2.0 tool was utilized to assess bias risk. Outcomes were analyzed using a random-effects meta-analysis, with subgroup analyses based on when glove changes occurred. Heterogeneity was evaluated through I² statistics. Results: Notably, changing gloves after the delivery of the placenta resulted in a significant reduction in wound infections (RR 0.37, 95% CI 0.26–0.53, p<0.001; I²=0%). However, glove changes before placental delivery did not show a significant effect (RR 0.62, 95% CI 0.15–2.49, p=0.5). Rates of endometritis remained unchanged (RR 0.91, 95% CI 0.75–1.10, p=0.332), although febrile morbidity was reduced overall (RR 0.57, 95% CI 0.35–0.93, p=0.024). High risk of bias was observed in performance/detection due to limited blinding, and some studies had incomplete data. Conclusion: Changing gloves after placental delivery during CS is linked to fewer wound infections and febrile morbidity, endorsing its adoption as an economical preventive strategy. However, due to methodological limitations, caution is advised in interpretation, while emphasizing potential actionable insights for infection management.
Keywords
Cesarean sections; Glove change
Introduction
Global Cesarean Section (CS) rates have risen from 7% in 1990 to 21% today, exceeding the WHO's ideal rate of 10%–15%. This trend is expected to continue, with projections indicating a global CS rate of 29% by 2030. Key reasons for C-sections include fetal distress (62% for emergency cases), previous cesarean (33% for elective), maternal request, and malpresentation, which all greatly influence the decision to perform the surgery [1].
Surgical Site Infection (SSI) following Cesarean Section (CS) occurs within 30 days post-surgery and affects the incision or deeper tissues [2]. SSI is common and leads to high maternal morbidity and mortality, patient dissatisfaction, prolonged hospital stays, and increased treatment costs [3, 4]. A variety of SSI rates were described after CS, including 2.94% in Libya [5], 4.3% in Uganda [6], and 18% in Pakistan [7].
Significant non-staphylococcal bacteria were identified on surgeons' gloves following Cesarean Sections (CS), likely originating from contact with the vaginal wall or endocervical canal. These contaminated gloves could introduce vaginal bacteria into the pelvic cavity and abdominal wall when reinserted into the endometrial cavity, potentially resulting in infections [8]. In the mid-1990s, small Randomized Controlled Trials (RCTs) investigated whether changing gloves during surgery could decrease CS-related infections, but no consensus was reached due to insufficient sample sizes [9-11]. Recent studies have reignited interest in this simple intervention [12, 13].
This systematic review and meta-analysis seek to thoroughly assess the existing evidence to determine whether changing gloves during Cesarean Sections decreases the risk of postoperative infections. The goal is to inform future research and support clinical decision-making.
Methodology
Protocol, search strategies, and sources
The protocol for this systematic review was based on PRISMA guidelines and registered a priori in PROSPERO in 2025 (CRD420251074484). Two authors (SN, KA) systematically searched MEDLINE, Scopus, Web of Science, and Cochrane Central for Randomized Controlled Trials (RCTs) on the effects of changing gloves intraoperatively on postoperative infectious morbidity from inception till 1st June 2025, with no language restrictions. In addition to the electronic database searches, we also hand-checked all reference lists of identified trials and other relevant articles. Our search strategy consisted of a pertinent combination of Medical Subject Headings (MeSH), core search terms, and synonyms for cesarean section, gloves, and infection.
Screening and study selection
Two review authors (SB and SA) utilized a reference management tool to import search results and remove duplicates. They independently screened the titles and abstracts of the retrieved studies for potential eligibility for full-text review. Subsequently, (EA and MA) double-checked the list of studies flagged as potentially eligible to ensure accuracy and completeness. NA and OZ performed title, abstract, and full-text screening, reaching consensus through discussion. Irrelevant studies, reviews, unpublished, or non-randomized studies were excluded. Any disagreements during this process were resolved through discussion and consultation with a third author (MA). A PRISMA flowchart visually summarizes the selection process, illustrating each step from initial identification to final inclusion.
Data collection and outcomes
Two authors (MA and AK) independently extracted data from the included trials using a standardized form, assessing each study's eligibility and quality. Any disagreements were resolved by a third author (AS). The primary outcome focused on the incidence of wound infections, defined according to the Centers for Disease Control and Prevention as superficial and deep Surgical Site Infections (SSIs). This outcome varied across studies, encompassing wound infection, serous drainage, induration, pus, and gaping. For secondary outcomes, we considered clinical endometritis (defined by elevated temperature and uterine tenderness) and febrile morbidity (persistent fever without a clear infection source) [14-16]. Additionally, subgroup analyses were conducted based on the timing of glove changes—either after the baby's delivery but before the placenta, or after placenta delivery but before closing the abdominal wall.
Risk of bias
Two authors (SN and KA) independently evaluated the risk of bias in the trials using the Cochrane Risk of Bias tool [17]. They assessed factors such as random sequence generation, allocation concealment, blinding of participants, outcome assessment, completeness of data, and selective reporting. Disagreements were resolved through discussion, and a third author (AS) was consulted if necessary. Trials with inadequate reporting were deemed to have an unclear risk of bias. This analysis was essential for interpreting findings, identifying potential sources of heterogeneity, and assessing the strength of the evidence.
Measures of effect
Data from the included trials were processed according to the Cochrane Handbook for Systematic Reviews of Interventions. Two authors (EA and NA) performed data analysis using RevMan software, with a third author (AS) consulted when disagreements arose. MedCalc® calculated risk ratios (RR) and 95% Confidence Intervals (CI) for all outcomes.
Handling missing data
Our goal was to analyze data intention-to-treat, meaning participants were evaluated according to their initial randomization groups. In instances of missing data, we utilized figures provided by the study authors.
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the effects of including or excluding trials with a high risk of bias. However, this was not always feasible due to the limited number of studies.
Assessment of heterogeneity and subgroup analysis
We assessed trial heterogeneity using the I2 index, categorized as low, moderate, or high. For high heterogeneity, subgroup analyses were conducted. A random-effects model aggregated trials, as effect estimates may vary due to sampling differences and intervention effects.
Assessment of publication bias
To assess publication bias and small-study effects, we used funnel plots and Egger's test in MedCalc® (version 19.6.4). Symmetrical plots indicated low bias risk, while asymmetry suggested otherwise, with low p-values indicating potential bias.
Results
Fig. 1. illustrates the literature search strategy. The database search resulted in eight articles for the systematic review, involving 2,168 women. The timing of glove changes varied: three Randomized Controlled Trials (RCTs) [9-11] changed gloves before placenta delivery (n=979), while four did so afterward (n=919) [13, 18-20]. One study assessed both methods in a three-arm trial (n=150) [12], with a subgroup analysis following (Tab. 1.).
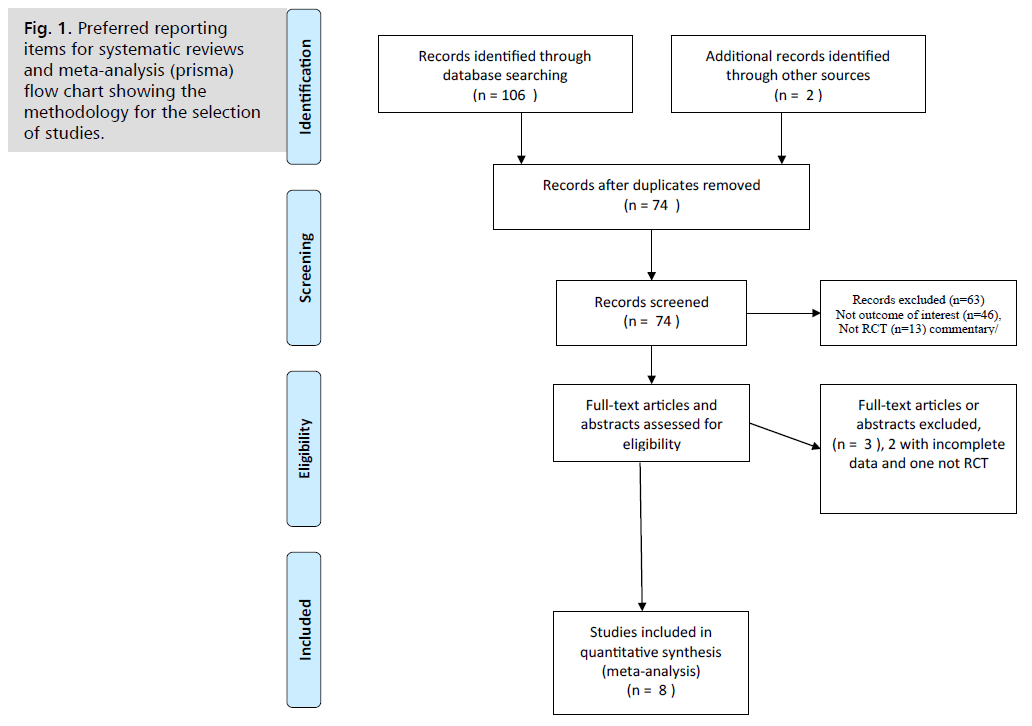
Fig. 1. Preferred reporting items for systematic reviews and meta-analysis (prisma) flow chart showing the methodology for the selection of studies.
| Study | Author | Publication | Study | Country | Study period | Sample size | Inclusion criteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year | design | |||||||||||
| 1 | Atkinson MW, et al [11] | 1996 | RCT | USA | 1993 to 1994 | 643 | ELCS/ EMCS | |||||
| 2 | Turrentine MA, | 1996 | RCT | USA | 1994 to | 108 | ELCS/ EMCS | |||||
| et al [9] | 1995 | |||||||||||
| 3 | Cernadas M, | 1998 | RCT | USA | 1995 | 108 | ELCS/ EMCS | |||||
| et al [10] | to | |||||||||||
| 1996 | ||||||||||||
| 4 | Szatámary PF, | 2004 | RCT | Hungary | Data not | 241 | CS | |||||
| et al [18] | available | |||||||||||
| (abstract) | ||||||||||||
| 5 | Ventolini G, | 2004 | RCT | USA | Data not | 92 | CS | |||||
| et al [19] (abstract) | available | |||||||||||
| 6 | Devoor A, | 2014 | RCT | India | Data not | 150 | CS | |||||
| et al [12] | available | |||||||||||
| 7 | Scrafford JD, | 2018 | RCT | USA | 2015 to | 486 | CS | |||||
| et al [13] | 2016 | |||||||||||
| 8 | Abbass MM, et al [20] | 2024 | RCT | Egypt | February 2023 to July 2023 | 220 | CS | |||||
| Exclusion criteria | Preoperative and intrapartum infection prophylaxis | Intervention | Comparison | Outcomes | Follow-up | |||||||
| Chorioamnionitis | prophylactic antibiotics (first-generation cephalosporin) | Glove change before SROP (164) or MROP (161) | No glove change and SROP (156) | Primary outcome: endometritis | No data | |||||||
| or MROP (162) | ||||||||||||
| Chorioamnionitis | Prophylactic antibiotics are administered after clamping the umbilical cord. | Glove change before MROP (113) | No glove change and MROP n-115 | Primary outcome: endometritis | 2 weeks postoperative | |||||||
| Multifetal pregnancies, urinary or respiratory infections | Prophylactic antibiotics are administered after clamping the umbilical cord. | Glove change before SROP (28) or MROP (27) | No glove change before SROP (27) or MROP (26) | 1ry outcome (postpartum febrile morbidity) | Till discharge | |||||||
| Incomplete data (abstract only) | Incomplete data (abstract only) | Charge gloves after SROP (151) | No glove change, MROP, and perioperative antibiotics (90) | 1ry outcome: endometritis | Incomplete data (abstract only) | |||||||
| Incomplete data (abstract only) | Incomplete data (abstract only) | Glove change after delivery of placenta (46) | No glove change (46) | 1ry outcome: SSSI. | Incomplete data (abstract only) | |||||||
| Morbid obesity, ROM, diabetes, and immunocompromised conditions | Prophylactic antibiotics are administered after clamping the umbilical cord. | 2 arms; Glove change after delivery of the fetus (50), Glove change after delivery of the placenta (50) | No glove change (50) | 1ry outcome (postpartum febrile morbidity) | Incomplete data | |||||||
| 2 ry: outcome:SSSI | ||||||||||||
| Obese women (BMI ≥ 30), anemic women, immunocompromised conditions, diabetes, multifetal pregnancy, emergency cesarean | Preoperative antibiotics | Change of gloves | No glove change | 1ry outcome: | Up to | |||||||
| after placental delivery (110) | -110 | SSSI | 4 weeks | |||||||||
| 2 ry postpartum febrile morbidity | post-CS | |||||||||||
| Multifetal pregnancies and | Preoperative antibiotics, | Change of gloves | No glove change | 1ry outcome: | 8 w post CS | |||||||
| emergency CS | before | -250 | outcome: 2 ry | |||||||||
| closure - | postpartum febrile morbidity | |||||||||||
| -236 | ||||||||||||
Tab. 1. Characteristics of included studies.
Risk of bias assessment revealed two studies [18, 19] had incomplete data, increasing their risk of bias. Fig. 2. shows a low risk of selection bias, as five studies randomized participants while three did not [12, 18, 19]. Four studies reported concealment [10, 11, 13, 20]. The risk of performance bias was relatively high, with only one study blinding outcome assessment for investigators [10] and another for patients [13]. Detection bias was similarly high, with only three studies having blinded outcomes [10, 12, 13]. Attrition bias risk was low, except for one trial [9]. Egger, et al. stated that publication bias evaluation was unreliable with fewer than 10 RCTs, which limited our assessment due to only five RCTs being included [21, 22].
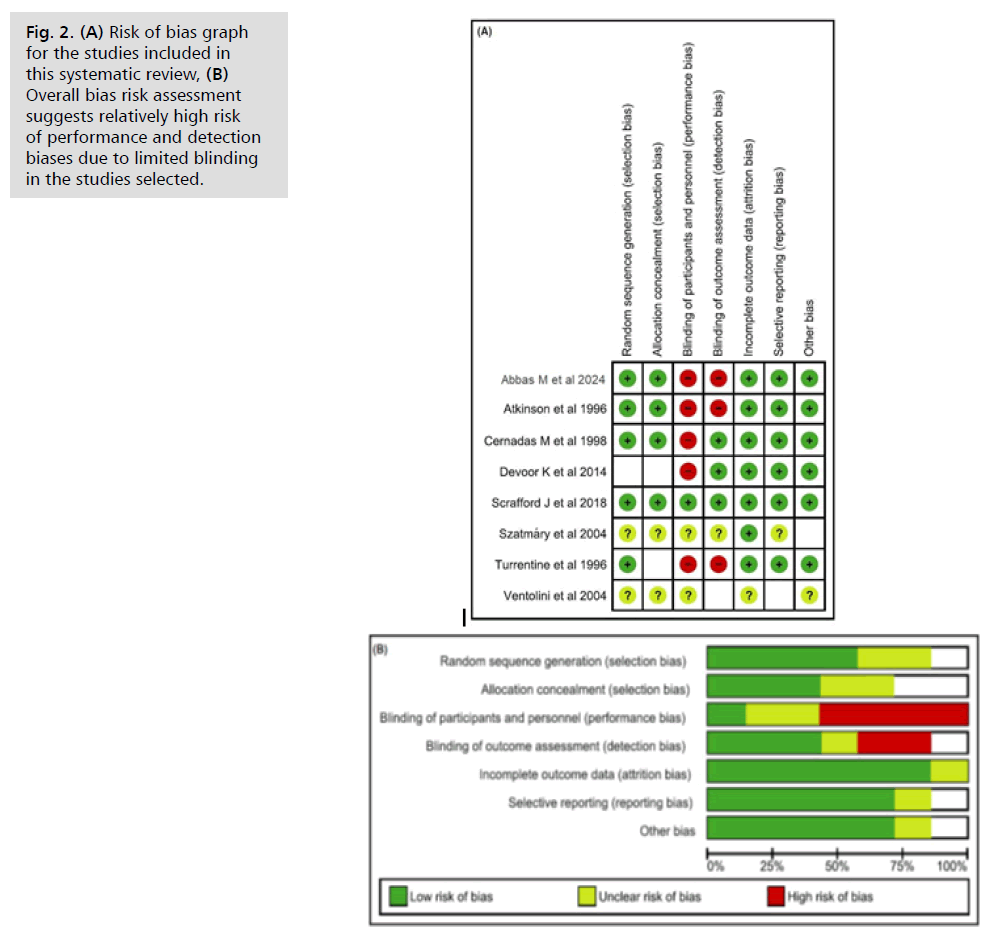
Fig. 2. (A) Risk of bias graph for the studies included in this systematic review, (B) Overall bias risk assessment suggests relatively high risk of performance and detection biases due to limited blinding in the studies selected.
Wound infective complications (Fig. 3.)
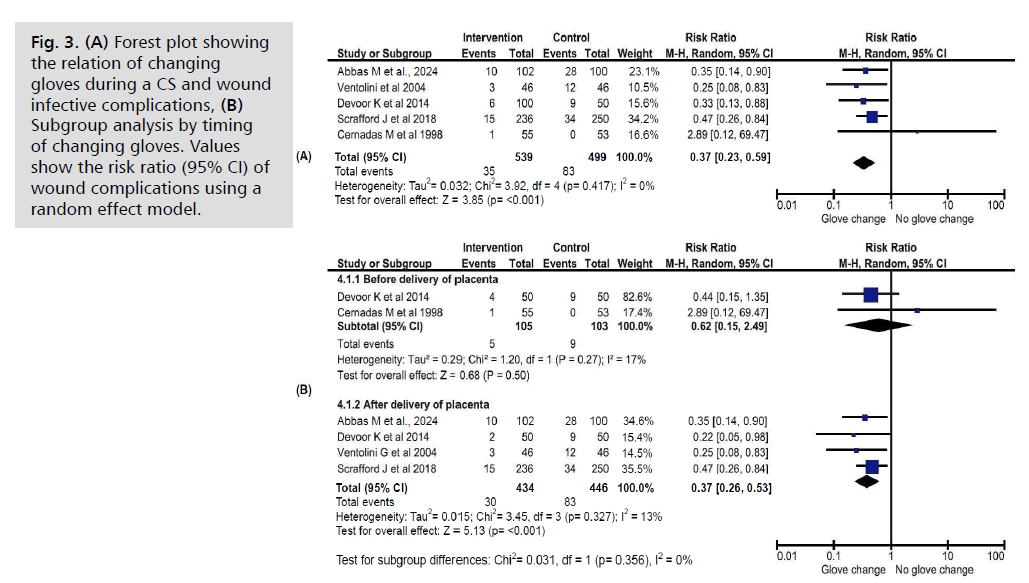
Fig. 3. (A) Forest plot showing the relation of changing gloves during a CS and wound infective complications, (B) Subgroup analysis by timing of changing gloves. Values show the risk ratio (95% CI) of wound complications using a random effect model.
Five studies [10, 12, 13, 19, 20] examined the rate of wound infections related to glove changing, including data from 1038 women. Of these, 51.9% (n=539) were in the glove-changing groups, and 47.73% (n=499) were in the control groups. The results showed that wound infections were significantly less common in the glove-changing groups (Relative Risk [RR] 0.37, 95% Confidence Interval [CI] 0.23–0.59, p<0.001). A subgroup analysis revealed that changing gloves after placenta delivery [12, 13, 19, 20] was associated with a reduced risk of wound infection (RR 0.37, 95% CI 0.26–0.53, p<0.001), whereas changing gloves before delivery [10, 12] did not show a significant effect (RR 0.62, 95% CI 0.15–2.49, p=0.5). The studies exhibited low heterogeneity (I2=0%). Even when focusing on interventions before delivery, heterogeneity remained low (I2=17%).
Endometritis (Fig. 4.)
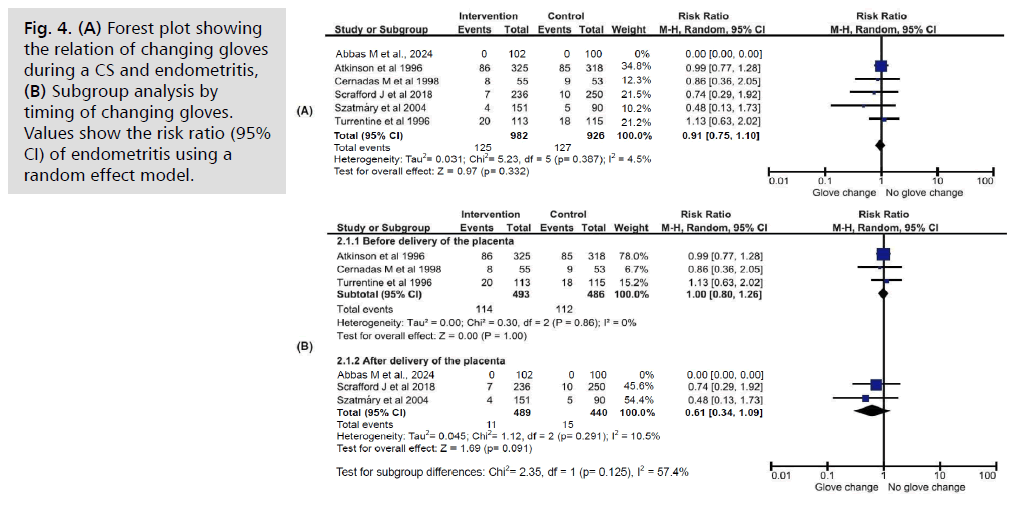
Fig. 4. (A) Forest plot showing the relation of changing gloves during a CS and endometritis, (B) Subgroup analysis by timing of changing gloves. Values show the risk ratio (95% CI) of endometritis using a random effect model.
Six studies [10, 11, 13, 18-20], involving a total of 1,908 women, investigated the occurrence of endometritis following glove changes during cesarean sections, compared to routine care. Slightly over half of the participants (51.5%, n=982) were in the glove-changing group, with the remaining 48.5% (n=926) in the control group. There was no significant difference in endometritis rates between the two groups (RR 0.91, 95% CI 0.75–1.10, p=0.332). Three studies evaluated glove changing before placenta removal [9-11], and three assessed it after delivery [13, 18, 20]. Subgroup analysis based on timing did not reveal any significant impact on endometritis incidence. Heterogeneity across studies was low (I2=4.5%).
Secondary outcome (2): Febrile morbidity (Fig. 5.)
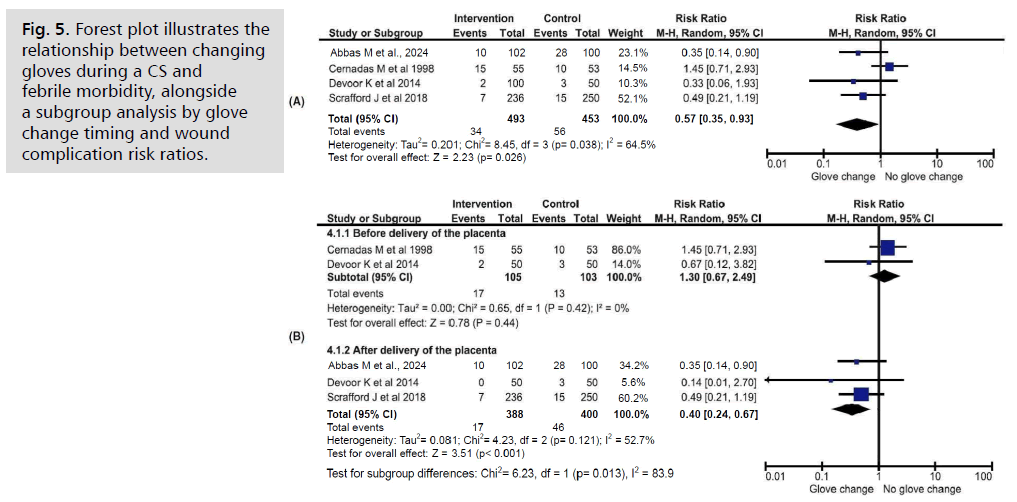
Fig. 5. Forest plot illustrates the relationship between changing gloves during a CS and febrile morbidity, alongside a subgroup analysis by glove change timing and wound complication risk ratios.
Four studies [10, 12, 13, 20] involving 946 women compared febrile morbidity rates based on whether gloves were changed during cesarean sections. The groups were similarly sized (intervention: 52.1%, n=493; control: 47.9%, n=453). A significant difference was found in postoperative febrile morbidity regardless of the timing of glove change (RR 0.57, 95% CI 0.35–0.93, p=0.024). Unlike the other outcomes, heterogeneity among these studies was moderate (I2=64.5%), but it decreased when the data were grouped based on the timing of the intervention.
Discussion
Postoperative infectious complications following Cesarean Sections (CS) remain a common issue, contributing significantly to maternal morbidity. These complications not only negatively impact the physical and emotional health of new mothers and their families but also impose considerable financial strain on healthcare systems [23].
For instance, within the UK's National Health Service, reducing Surgical Site Infections (SSI) related to CS could cut the costs of managing postoperative infections by up to £4,000 per case, thanks to shorter hospital stays and reduced antibiotic use. As such, there is an urgent need for simple, cost-effective interventions that can be easily integrated into clinical practice to help mitigate these risks [24, 25]. Our systematic review indicates that small but targeted modifications to current procedures could be instrumental in this global effort.
Our findings and their interpretation
As regards the wound infection, the analysis demonstrated a clinically meaningful reduction in wound infections with intraoperative glove changes (RR 0.37, *p*<0.001), driven by interventions performed *after* placental delivery (RR 0.37, *p*<0.001). No significant effect was observed when gloves were changed pre-placental delivery (RR 0.62, *p*=0.5). Consistency across studies (I2=0%) underscores the robustness of this timing-dependent association. High performance/detection bias (limited blinding) and reliance on unblinded assessments weaken causal inferences. Still, the findings suggest that post-placental glove changes may mitigate bacterial contamination during wound closure, aligning with mechanistic hypotheses about intraoperative contamination pathways.
Endometritis rates showed no significant difference between groups (RR 0.91, p=0.332), regardless of glove-change timing, with low heterogeneity (I2=4.5%) supporting this null effect. Prophylactic antibiotics, widely administered across studies, may explain the lack of divergence in localized uterine infections. In contrast, febrile morbidity was significantly reduced (RR 0.57, p=0.024), though moderate heterogeneity (I2=64.5%) suggests variability in outcome definitions or measurement. Subgroup analysis by timing resolved some heterogeneity, implying methodological differences (e.g., fever criteria) influenced results. This divergence highlights that glove changes may impact systemic inflammatory responses more than localized infections.
The risk of bias assessment identified high performance/detection bias due to limited blinding in most studies, reducing confidence in subjective outcomes like endometritis. Incomplete data in two trials [18, 19] further weakened reliability for those endpoints. Selection bias was largely mitigated through randomization and allocation concealment in five studies. However, small sample sizes and an inability to assess publication bias (fewer than 10 RCTs) limit generalizability. While wound infection results are statistically robust, the high risk of bias in blinding and modest study scales temper the strength of conclusions about causality.
The underlying mechanisms are not fully understood, but it is hypothesized that intraoperative glove changing might effectively contain local infections by reducing contamination of the wound with vaginal flora during surgery. Since low-grade fever after surgery is often a physiological response rather than an infection, changing gloves may not significantly impact febrile morbidity. Similarly, endometritis is primarily influenced by factors occurring before or during labor—such as vaginal dysbiosis, prolonged rupture of membranes, or multiple vaginal examinations—suggesting that intraoperative glove changes might not address these pre-existing risks.
Clinical implications
This review advocates for intraoperative glove changes after placental delivery during Cesarean Sections to reduce wound infections (RR 0.37), supporting contamination-reduction hypotheses. Though no significant impact on endometritis was observed, prophylactic antibiotics may help mitigate uterine infection risks. Additionally, a reduction in febrile morbidity (RR 0.57) suggests broader systemic benefits. Despite high bias risks and limited sample sizes, these findings are clinically relevant, making post-placental glove changes a low-cost adjunct to infection-control practices in high-risk settings.
Strengths and limitations of our review
Our strengths in this review adhered rigorously to PRISMA guidelines, ensuring methodological transparency and reproducibility in study selection. Including Randomized Controlled Trials (RCTs) enhanced internal validity, while subgroup analyses (e.g., glove-change timing) provided nuanced insights into intervention efficacy. Risk of bias was systematically assessed using Cochrane criteria, highlighting methodological strengths (e.g., low selection bias in five studies) and weaknesses. The wound infection findings were statistically robust, with low heterogeneity (I2=0%) and clinically significant effect sizes (RR 0.37, *p*<0.001), particularly for post-placental glove changes. Consistency across endpoints like febrile morbidity (RR 0.57, *p*=0.024) further supports the plausibility of glove changes influencing systemic outcomes. The focus on intraoperative protocols addresses an understudied aspect of surgical site infection prevention, offering actionable data for clinical practice.
This review, guided by PRISMA standards and including gray literature, has several important limitations. The inclusion of only eight studies with modest sample sizes (n=92–220) limited statistical power, especially for subgroup analyses. The focus on gray literature also introduced methodological inconsistencies, as some studies lacked essential details like antibiotic protocols and outcome definitions, complicating comparisons. Many studies ranged over three decades and were published before CONSORT standards, resulting in incomplete methodological descriptions that diminished quality assessment. Performance and detection biases were prevalent, as most trials did not blind participants, surgeons, or assessors, potentially skewing subjective outcomes such as endometritis. Additionally, key confounding factors were reported inconsistently, and concerns over attrition bias arose due to the lack of intention-to-treat analyses. Heterogeneity in the definition of febrile morbidity (I2=64.5%) further complicated interpretation, although subgroup analyses helped clarify some issues. Most studies originated from high-income settings, which may restrict applicability in lower-resource contexts. Differences in the timing of antibiotic administration across trials could also have influenced infection outcomes. These factors highlight the necessity for more rigorous and standardized Randomized Controlled Trials (RCTs) to confirm findings and elucidate mechanisms.
Recommendations for further reviews
Future reviews should focus on large, multicenter Randomized Controlled Trials (RCTs) with standardized outcome measures, like febrile morbidity, and rigorous blinding to minimize bias. Research in low-resource settings and diverse populations is essential for assessing generalizability. Exploring how glove changes, antibiotic timing, and contamination routes affect outcomes could shed light on underlying mechanisms. Additionally, evaluating cost-effectiveness and long-term effects is crucial. Including non-English studies and gray literature can help reduce publication bias. Ultimately, while glove changing after placental delivery appears beneficial for reducing wound complications post-Cesarean section, more research is needed to support broader implementation.
Conclusion
Post-placental glove changes during Cesarean Sections significantly reduce wound infections and febrile morbidity, though no effect on endometritis was observed. Despite clinical relevance, high bias risks and limited sample sizes caution interpretation. These findings highlight a simple, cost-effective intervention for infection control, meriting further validation in robust trials.
References
- Angolile CM, Max BL, Mushemba J, et al. Global increased cesarean section rates and public health implications: A call to action. Health Sci Rep. 2023;6(5):e1274.
- Shacho E, Yilma D, Goshu AT, et al. Incidence and risk factors of surgical site infection following cesarean section: A prospective cohort study at Jimma University Medical Center. BMC Infect Dis. 2025;25(1):457.
- Bogdanović G, Cerovac A. Bacterial causes and antibiotics susceptibility profile of surgical site infection following cesarean section. Clin Exp Obstet Gynecol. 2022;49(4):1-4.
- Zejnullahu VA, Isjanovska R, Sejfija Z, et al. Surgical site infections after cesarean sections at the University Clinical Center of Kosovo: Rates, microbiological profile and risk factors. BMC Infect Dis. 2019;19(1):1-9.
- Alkout T, Alkout AM, Rasheed E, et al. Surgical site infections prevalence among caesarean section patients. Iberoam J Med. 2024;7(1):11-16.
- Hussein MA, Sutiningsih D, Ntambi S, et al. The prevalence and the association of antibiotic practices with surgical site infection following cesarean section. J Epidemiol Kesehatan Komunitas. 2024;9(1):26-33.
- https://bcsrj.com/ojs/index.php/bcsrj/article/view/1309
- Yancey MK, Clark P, Duff P. The frequency of glove contamination during cesarean delivery. Obstet Gynecol. 1994;83:538-542.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4:16-19.
- Cernadas M, Smulian JC, Giannina G, et al. Effects of placental delivery method and intraoperative glove changing on postcesarean febrile morbidity. J Matern Fetal Med. 1998;7:100-104.
- Atkinson MW, Owen J, Wren A, et al. The effect of manual removal of the placenta on post-cesarean endometritis. Obstet Gynecol. 1996;87:99-102.
- Devoor A, Roopadevi GHM. Effects of intraoperative "changing glove technique" on post-cesarean infectious morbidity. SJAMS. 2014;2:3118-3122.
- Scrafford JD, Reddy B, Rivard C, et al. Effect of intra-operative glove changing during cesarean section on post-operative complications: A randomized controlled trial. Arch Gynecol Obstet. 2018;297:1449-1454.
- Saeed KBM, Greene RA, Corcoran P, et al. Incidence of surgical site infection following cesarean section: a systematic review and meta-analysis protocol. BMJ Open. 2017;7:e013037.
- Onyekwelu I, Yakkanti R, Protzer L, et al. Surgical wound classification and surgical site infections in the orthopedic patient. J Am Acad Orthop Surg Glob Res Rev. 2017;1:e022.
- https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration. 2011.
- Szatmáry PF, Papp KG, Gáspár A, et al. Intraoperative glove change and assisted spontaneous placental delivery as methods to decrease the risk of post-cesarean endometritis. Magy Nőorv Lapja. 2004;67:225-232.
- Ventolini G, Neiger R, McKenna D. Decreasing infectious morbidity in cesarean delivery by changing gloves. J Reprod Med. 2004;49:13-16.
- Abbas MM, Hamed El-Feky A, Abd El-Aal FH. Impact of changing sterile glove at the time of wound closure to reduce surgical site infection in women undergoing elective cesarean section: a prospective randomized controlled clinical trial. HJOG. 2024;23(3):171-181.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 2015;14:1-16.
- Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113-2126.
- Kayembe AT, Kapuku SM. Postoperative maternal complications of cesarean section: A cross-sectional study at the Provincial General Hospital of Kananga in the Democratic Republic of Congo. Pan Afr Med J. 2024;47:23.
- Stanirowski PJ, Davies H, McMaster J, et al. Cost-effectiveness of a bacterial–binding dressing to prevent surgical site infection following cesarean section. J Wound Care. 2019;28:222-228.
- Wloch C, Van Hoek AJ, Green N, et al. Cost-benefit analysis of surveillance for surgical site infection following cesarean section. BMJ Open. 2020;10:e036919.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Salwa Neyazi1, Khalid Akkour1, Nada Alayed1, Omar Zidan1, Eman Al Shehri1, Shadan Binsaeedan1, Mashael Alshebly1, Mustafa Smisim1, Ahmed Sherif1,2, Alhassan Khedr3*, Sondos Al Hawamdeh4, Mohammed A. Alatawi5,6, Mohammad Atlam, Amal Kalifa7 and Karim Abdelsalam82Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
3Department of Obstetrics and Gynecology, Doctor Sulaiman Alhabib Medical Group, Riyadh, Saudi Arabia
4Department of Obstetrics and Gynecology, Faculty of Medicine, Tabuk University, Cairo, Egypt
5Anesthesia Registrar, Department of Anesthesia, Saudi German Hospital, Makkah, Saudi Arabia
6PHD in Anesthesia and Intensive Care, El Azhar University, Cairo, Egypt
7Department of Obstetrics and Gynecology, Faculty of Medicine, Elimam Elmahdi University, Sudan
8Department of Obstetrics and Gynecology, Faculty of Medicine, Helwan University, Helwan, Egypt
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.