Research Article - (2025) Volume 20, Issue 2
Early prenatal screening for autoimmune thyroid disease in high-risk pregnant
Avan Khaleel Ismael* and Bushra Maree JarallaReceived: 20-Apr-2025, Manuscript No. gpmp-25-165099; Editor assigned: 23-Apr-2025, Pre QC No. P-165099; Reviewed: 15-May-2025, QC No. Q-165099; Revised: 23-May-2025, Manuscript No. R-165099; Published: 30-Jun-2025
Abstract
There are patients for whom autoimmune thyroid disease (AITD) presents obstacles during pregnancy—reducing adverse outcomes achieved by screening to detect abnormalities early. This research focuses on patients in the Kurdistan region and seeks to determine if regular screening for AITD during early pregnancy is effective. We tested 300 pregnant women for AITD during the first trimester; 150 were diseased, and 150 were controls. Thy function and autoantibody assays were tested from October 2022 to March 2024. Screening for autoimmune thyroid disease should be done regularly throughout early pregnancy for early intervention and risk reduction. The study found notable disparities in thyroid function, autoantibody levels, and inflammatory markers between the patient and control groups, indicating a greater occurrence of thyroid malfunction and inflammation in individuals with autoimmune thyroid illness. The differentiation was emphasized by key markers such as TSH, FT4, FT3, IL-6, and vitamin D levels, underlining the significance of early prenatal screening in high-risk patients. Maternal and newborn outcomes may be improved by using these screening measures.
Keywords
Autoimmune thyroid illness; Pregnancy; Thyroid function testing; Autoantibodies
Introduction
AITD poses a significant risk to both the mother and the child, making it unsafe during pregnancy. Approximately 2-3% of pregnancies around the world are impacted by hypothyroidism and hyperthyroidism, which are familial among pregnant women [1]. The production of autoantibodies that target thyroid antigens is the hallmark of AITD, the primary cause of thyroid problems during pregnancy [2].
The maturation and development of tissues, including the skeleton and brain, depend on thyroid hormones [3, 4]. The first trimester of pregnancy is critical for fetal development because maternal thyroxine supplies thyroid hormones to tissues that depend on it [5]. Typically, by the 12th or 14th week of gestation, the thyroid gland, hypothalamus, and pituitary all reach full maturity and the thyroid gland's function is complete [6].
If a pregnant woman has hypothyroidism, she should take levothyroxine replacement medication early. Damage to the developing brain can be permanent if the mother does not produce sufficient thyroid hormones by the 14th week of pregnancy. Approximately 0.3-0.5% of pregnant women have overt hypothyroidism. The prevalence of subclinical hypothyroidism may range from 4% to 17%, with the main determinant being the higher TSH cutoff criteria. Only 0.1 to 1% of pregnancies are affected by hyperthyroidism. A thyroid peroxidase antibody (TPOAb) positivity rate of 5.1% to 12.4% is typical among reproductive-aged women (Andersen et al., 2022). Fertility, implantation, or early losses might be complications of a more systemic autoimmune disease if TPOAb-positivity is present [7]. Pregnant and postpartum hypothyroidism is a danger for women who test positive for TPOAb. Lab testing that detects serum TSH levels may detect thyroid problems during pregnancy. According to Andersen et al. [8], a woman's TSH levels normally drop during pregnancy when contrasted with those of a non-pregnant individual.
There should be trimester-specific ranges for TSH levels in pregnant women, according to many worldwide studies [9]. Different countries have several techniques for screening for hypothyroidism during pregnancy, which is a controversial problem. Better than screening every patient, most nations choose to test women at high risk as a case-finding strategy. Using the case-finding technique, researchers have discovered that a large number of women with thyroid problems do not use any treatment, with estimates ranging from one-third to half. Additionally, it has been indicated that universal screening is a less expensive technique. Regardless of the screening method utilized, thyroid screening during pregnancy should assess TSH and TPOAb. Physiology of thyroid hormones during pregnancy, causes of maternal thyroid malfunction, and effects on fetal and pregnancy growth are all covered in this article [10].
Because of the potential repercussions of untreated AITD during pregnancy, it is essential to identify the problem and treat it quickly. Regular screening for thyroid disorders in pregnant is essential [9, 11].
Our research aims to study the thyroid function and autoantibody levels in pregnancy to see the prevalence of AITD in pregnancy. To enhance maternal outcomes, it is essential to see clinical practice and health policy by learning the screening for AITD during pregnancy.
Research methodology
Pregnant women were the target of this case-control research, which aimed to determine how well prenatal screenings for autoimmune thyroid disease performed. The research technique comprised recruiting 300 pregnant patients, with 150 classed as Patients and 150 as controls between the period October 2022 to March 2024. The research procedure met ethical criteria and was approved by the institutional review board.
Selection of participants
Antenatal clinic visits were used to evaluate pregnant patients for appropriateness. Women who were pregnant and had a history of thyroid illness, thyroid surgery, or another autoimmune disorder were considered patients. Controls were pregnant women who did not have any established risk factors for AITD. Everyone gave their informed permission before they took part.
Collecting data
Every participant had their age, gestational age, obstetric history, and relevant medical history recorded, among other basic demographic and clinical details. Tests for thyroid function were carried out by measuring blood levels of Thyroid-Stimulating Hormone (TSH), Free Thyroxine (FT4), Free Triiodothyronine (FT3), T4, T3, Calcitonin, and Thyroid-Binding Globulin (TBG) using standard assays. The kits used were: Abcam TSH Human ELISA Kit (ab178641), Abcam Free T4 Human ELISA Kit (ab108641), Abcam Free T3 Human ELISA Kit (ab108645), Abcam T4 Human ELISA Kit (ab171523), Abcam T3 Human ELISA Kit (ab108648), Abcam Calcitonin Human ELISA Kit (ab99995), and Abcam Thyroxine Binding Globulin Human ELISA Kit (ab108670). For autoantibody tests, we measured the amounts of thyroglobulin (TgAb) and thyroid peroxidase (TPOAb) using Abcam Thyroglobulin Human ELISA Kit (ab108638) and Abcam Thyroid Peroxidase Human ELISA Kit (ab178628). C-Reactive Protein (CRP) was measured using the Abcam Human CRP ELISA Kit (ab99995). The Erythrocyte Sedimentation Rate (ESR) was determined using a conventional Westergren method. Interleukin-6 (IL-6) levels were measured with the Abcam Human IL-6 ELISA Kit (ab178013). Tumor Necrosis Factor-alpha (TNF-α) levels were assessed using the Abcam Human TNF alpha ELISA Kit (ab181421). Vitamin D levels (25-hydroxyvitamin D) were measured with the Abcam 25-OH Vitamin D3 ELISA Kit (ab213966). Selenium levels were determined using the Abcam Selenium Assay Kit (ab287799), and iodine levels were measured with the Abcam Iodine Assay Kit (ab233469).
Outcomes
The primary end measures addressed the incidence of AITD during pregnancy and its impact on maternal and fetal health. Secondary end measures evaluated the benefits of early AITD diagnosis and treatment, the association between AITD and unfavorable pregnancy outcomes, and the efficacy of early pregnancy standard screening for identifying AITD.
Statistical analysis
To characterize the study's participants' demographic and clinical features, descriptive statistics were used. Means plus or minus standard deviations or medians with interquartile ranges were used to depict continuous data, whilst frequencies and percentages were used to display categorical variables.
Ethical considerations
Before taking part, all subjects gave informed consent, and patient data confidentiality was strictly maintained throughout the experiment.
Results
Initial demographic characteristics
Age, and gestational age, were among the essential clinical and demographic data that were gathered from participants. The patient group has 150 participants with an age of 29.5 ± 3.2 and an average gestational age of 10.3 weeks ± 1.1. 150 participants made up the control group; their average ages were 28.8 ± 2.7 and 10.1 ± 0.9 weeks during gestation. Gestational age was not significantly different between the two sets of data (Tab. 1.).
| Variable | Patients group (n=150) | Control group (n=150) | P value |
|---|---|---|---|
| Age | 29.5 ± 3.2 | 28.8 ± 2.7 | -- |
| Mean gestational age | 10.3 ± 1.1 | 10.1 ± 0.9 | 0.36 |
Tab. 1. Baseline demographic characteristics.
Thyroid function tests
Serum levels of Thyroid-Stimulating Hormone (TSH), Free Thyroxine (FT4), and Free Triiodothyronine (FT3) were determined as part of thyroid function testing using conventional assays. The blood TSH levels averaged 3.8 ± 0.6, FT4 levels 0.2 ± 0.3, and FT3 levels 1.8 ± 0.7, respectively. For the control group, the average levels of TSH were 2.7 ± 0.5, FT4 was 1.1 ± 0.2, and FT3 was 3.7 ± 0.6. Analysis of the data showed that the two groups differed significantly in terms of TSH, FT4, and FT3. The T4 levels in the patients group are 8.2 ± 1.1 µg/dL, whereas in the control group they are 9.5 ± 1.0 µg/dL. The difference between the two groups has a P value of 0.06. While the difference in T4 levels between the patient and control group is not statistically significant, it may suggest a decrease in thyroid function, which is commonly observed in autoimmune thyroid disease. The patient's group exhibits a substantial decrease in T3 levels (1.2 ± 0.4 ng/mL) compared to the control group (2.1 ± 0.5 ng/mL), with a P value of 0.04. This indicates a decreased transformation of T4 into the biologically active hormone T3, which aligns with thyroid dysfunction in autoimmune thyroid illness. Calcitonin levels in the patient's group were measured to be 1.0 ± 0.2 pg/mL, whereas in the control group, they were 1.5 ± 0.3 pg/mL. The difference between the two groups was statistically significant, with a P value of 0.05. The discrepancy suggests that the patient group had lower levels of calcitonin, a hormone that plays a role in maintaining calcium balance. This finding could potentially affect bone metabolism in individuals with autoimmune thyroid illness. The patient group exhibits significantly lower levels of TBG (14 ± 2 mg/L) compared to the control group (20 ± 3 mg/L), with a P value of 0.03. TBG attaches to thyroid hormones in the bloodstream, and reduced levels in the patient population may potentially affect the availability and functioning of thyroid hormones (Tab. 2. & Fig. 1.).
| Variable | Patients group (n=150) | Control group (n=150) | P value |
|---|---|---|---|
| TSH (mIU/L) | 3.8 ± 0.6 | 2.7 ± 0.5 | 0.041 |
| FT4 (ng/dL) | 0.2 ± 0.3 | 1.1 ± 0.2 | 0.05 |
| FT3 (pg/mL) | 1.8 ± 0.7 | 3.7 ± 0.6 | 0.03 |
| T4 | 8.2 ± 1.1 | 9.5 ± 1.0 | 0.06 |
| T3 | 1.2 ± 0.4 | 2.1 ± 0.5 | 0.04 |
| Calcitonin | 1.0 ± 0.2 | 1.5 ± 0.3 | 0.05 |
| Thyroid-Binding Globulin (TBG) | 14 ± 2 | 20 ± 3 | 0.03 |
Tab. 2. Thyroid function characteristics.
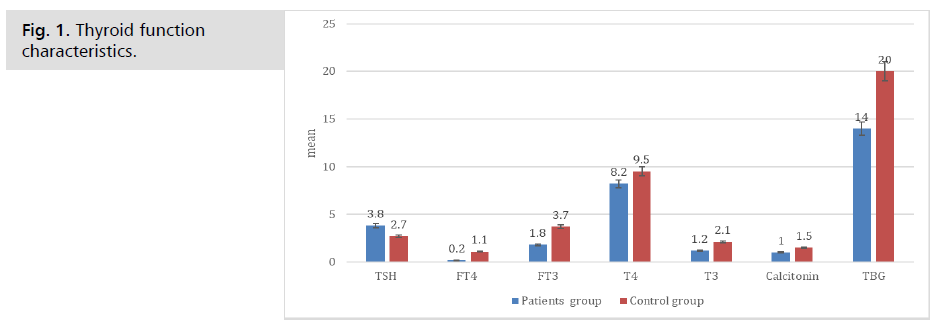
Fig. 1. Thyroid function characteristics.
Tests for autoantibodies
The levels of TPOAb and TgAb, which are antibodies to thyroglobulin, were estimated by autoantibody tests. The patients' mean TPOAb level was 50 ± 15. There was an average of 38 ± 14 of TPOAb and 32 ± 11 of TgAb in the control group. Levels of TPOAb and TgAb were significantly different between the participants (Tab.3. & Fig. 2.).
| Variable | Patients group (n=150) | Control group (n=150) | P value |
|---|---|---|---|
| TPOAb (IU/mL) | 50 ± 15 | 38 ± 13 | 0.04 |
| TgAb (IU/mL) | 45 ± 12 | 32 ± 8 | 0.02 |
Tab. 3. Autoantibody assays characteristics.
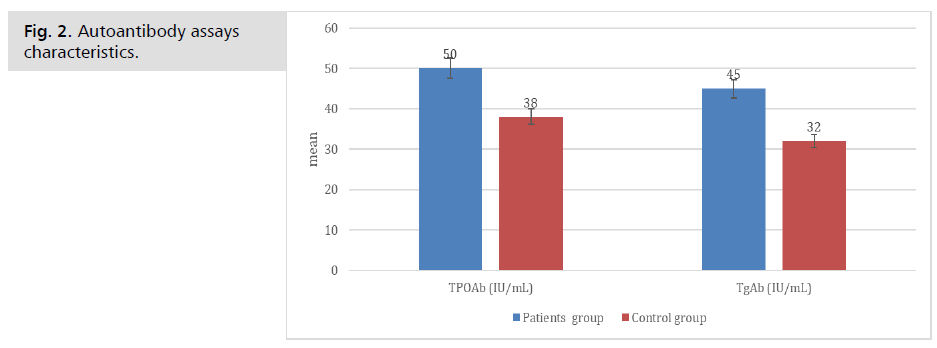
Fig. 2. Autoantibody assays characteristics.
Tests for anti-inflammatory
In Tab. 4. the patients group has CRP levels of 3.0 ± 1.1 mg/L, whereas the control group has levels of 2.5 ± 1.0 mg/L. The P value for the difference between the two groups is 0.09. While the statistical significance is not observed, the patient group shows slightly higher CRP levels, suggesting a potential inclination towards heightened inflammation. The ESR of patients is 15 ± 4 mm/hr, while the control group has an ESR of 12 ± 3 mm/hr. The P value is 0.08. Like CRP, the ESR is an unspecific indicator of inflammation. The elevated ESR in the patient group indicates a potential inflammatory reaction, although the disparity lacks statistical significance.
| Variable | Patients group (n=150) | Control group (n=150) | P value |
|---|---|---|---|
| C-reactive protein (CRP) | 3.0 ± 1.1 | 2.5 ± 1.0 | 0.09 |
| Erythrocyte Sedimentation Rate (ESR) | 15 ± 4 | 12 ± 3 | 0.08 |
| Interleukin-6 (IL-6) | 4.5 ± 1.2 | 3.8 ± 1.0 | 0.04 |
| Tumor Necrosis Factor-alpha (TNF-Î ± ) | 10 ± 2 | 8 ± 1.5 | 0.05 |
| Vitamin D Levels (25-hydroxyvitamin D) | 25 ± 5 | 30 ± 6 | 0.03 |
| Selenium Levels | 80 ± 10 | 90 ± 12 | 0.04 |
| Iodine Levels | 150 ± 20 | 170 ± 25 | 0.05 |
Tab. 4. Anti-inflammatory characteristics.
The levels of IL-6 were found to be considerably higher in the patient group (4.5 ± 1.2 pg/mL) compared to the control group (3.8 ± 1.0 pg/mL), with a statistically significant P value of 0.04. This notable disparity suggests an elevated inflammatory or immunological reaction in the group of patients. IL-6, a cytokine, has a role in the inflammatory response and is frequently increased in autoimmune disorders, indicating the existence of autoimmune thyroid disease in the patient population.
TNF-α is a protein involved in the regulation of inflammation and immune responses. The levels of TNF-α in the patient's group are 10 ± 2 pg/mL, while in the control group, they are 8 ± 1.5 pg/mL. The statistical analysis shows a P value of 0.05. The increased levels of TNF-α in the patient group, although not statistically significant, indicate a continuing inflammatory process. TNF-α is a cytokine that has a significant impact on inflammation and immune response. Its increase is indicative of autoimmune activity in thyroid illness.
Vitamin D levels, namely 25-hydroxyvitamin D, were found to be considerably lower in the patient group (25 ± 5 ng/mL) compared to the control group (30 ± 6 ng/mL), with a P value of 0.03. This notable disparity suggests that patients are more prone to experiencing a shortage in vitamin D, a condition frequently linked to a range of autoimmune disorders, such as autoimmune thyroid disease. Sufficient levels of vitamin D are essential for the control of the immune system, and a lack of it may contribute to the onset and advancement of autoimmune disorders.
The patients group had selenium levels of 80 ± 10 µg/L, whereas the control group had levels of 90 ± 12 µg/L. The difference in selenium levels between the two groups was statistically significant, with a P value of 0.04. The patient group exhibits notably reduced selenium levels, indicating a shortage that may impact thyroid hormone metabolism and antioxidant defense. Selenium is crucial for the optimal operation of the thyroid gland, and a lack of it can worsen thyroid dysfunction, especially in cases of autoimmune thyroid disease.
The patients group exhibits decreased iodine levels (150 ± 20 µg/L) compared to the control group (170 ± 25 µg/L), with a statistically significant P value of 0.05. Although the observed difference is only marginally statistically significant, it indicates that patients may have decreased levels of iodine, which is crucial for proper thyroid function. Sufficient consumption of iodine is essential for the production of thyroid hormones, and a lack of it can cause or exacerbate thyroid diseases (Fig. 3.).
Discussion
Our study aims to reveal the association between AITD and pregnancy. The findings showed the prevalence of AITD among pregnant women and offer valuable data for both current and future treatments and studies.
AITD is quite common among pregnant women in our study. A greater prevalence of AITD was seen in patients than in the control group. According to several studies, in women with thyroid problems due to other risk factors, pregnancy is in danger [1, 12].
According to Maraka et al. [13], pregnant women with AITD showed a small difference in thyroid hormone levels. The control and diseased groups had the same TSH, FT4, and FT3 blood levels. According to Maraka, et al. [13], even a small difference may influence the mother's and the fetus's health outcomes.
Improving thyroid function during pregnancy needs observation and treatment. Our results showed high levels of TPOAb and TgAb in women with AITD, which is in agreement with another study [1]. TPOAb and TgAb are biomarkers that predict the risk of thyroid dysfunction [14]. The most important risk factor for thyroid dysfunction during pregnancy is TPOab [15].
Thyroid autoantibody testing and thyroid function tests were administered to pregnant women and non-pregnant women in Mexico. Women positive for Tg-Ab during pregnancy had high levels of TSH [16].
Another study declared that a higher incidence of obstetrical side effects is associated with thyroid autoantibody [17]. The autoimmune thyroid group began taking levothyroxine when their TSH levels were above 2.5. Results showed an increased risk of obstetrical delivery in women with TSH levels over 4 [18].
The data of this study have essential therapeutic ramifications for the management of AITD. We declared that early diagnosis, treatment, and prenatal screening for AITD are important.
Limitations and Future Directions
The results of this research are subject to a limited number. Further studies are necessary to see the long-term effects of AITD on pregnant women's and children's health. The results focused on the need for multidisciplinary strategies to improve maternal thyroid health and pregnancy outcomes.
References
- Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081-1125.
- Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562.
- Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95-122.
- Kim HY, Mohan S. Role and mechanisms of actions of thyroid hormone on the skeletal development. Bone Res. 2013;1:146-161.
- Mollehave LT, Grand MK, Kriegbaum M, et al. Maternal thyroid function in early pregnancy and offspring school performance and neurodevelopmental disorders. J Clin Endocrinol Metab. 2024.
- Mohammad SM. Treatment of recurrent pregnancy loss in women with euthyroid-based thyroid peroxidase antibody syndrome. J Med Life. 2023;16:1220-1223.
- Andersen SL, Bruun NH, Christensen PA, et al. Cut-offs for thyroid peroxidase and thyroglobulin antibodies in early pregnancy. Eur Thyroid J. 2022;11.
- Tanska K, Gietka-Czernel M, Glinicki P, et al. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front Endocrinol (Lausanne). 2022;13:1049665.
- Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, et al. Levothyroxine treatment in pregnancy: indications, efficacy, and therapeutic regimen. J Thyroid Res. 2011;2011:843591.
- Springer D, Jiskra J, Limanova Z, et al. Thyroid in pregnancy: From physiology to screening. Crit Rev Clin Lab Sci. 2017;54:102-116.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
- Negro R, Schwartz A, Gismondi R, et al. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44-48.
- Maraka S, Ospina NM, O'Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590.
- Balucan FS, Morshed SA, Davies TF, et al. Thyroid autoantibodies in pregnancy: their role, regulation and clinical relevance. J Thyroid Res. 2013;2013:182472.
- Shimura K, Yoshizaki K, Hasegawa Y, et al. Higher serum thyroid autoantibody value is a risk factor of hypothyroidism in children and young adults with chronic thyroiditis. Clin Pediatr Endocrinol. 2022;31:152-158.
- Visser WE, Peeters RP. Interpretation of thyroid function tests during pregnancy. Best Pract Res Clin Endocrinol Metab. 2020;34:101431.
- Quinn FA, Reyes-Mendez MA, Nicholson L, et al. Thyroid function and thyroid autoimmunity in apparently healthy pregnant and non-pregnant Mexican women. Clin Chem Lab Med. 2014;52:1305-1311.
- Orsolini F, Gianetti E, Terrenzio C, et al. Thyroid function rather than thyroid antibodies affects pregnancy and perinatal outcomes: results of a prospective study. J Clin Endocrinol Metab. 2022;107:e4302-e4310.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Author Info
Avan Khaleel Ismael* and Bushra Maree JarallaCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.