Research - (2022) Volume 17, Issue 2
Correlation between fetal ductus venosus doppler velocimetry, pulsitility index (MCA/UA ratio) and amniotic fluid index in prediction Of perinatal outcome in preeclamptic pregnancies
Mohamed I Taema*, Nada Alayed#, Salwa Neyazi, Ibrahim Ali and Amr SobhyReceived: 14-Jun-2022, Manuscript No. gpmp-22-66590; Editor assigned: 15-Jun-2022, Pre QC No. P-66590; Reviewed: 21-Jun-2022, QC No. Q-66590; Revised: 24-Jun-2022, Manuscript No. R-66590; Published: 30-Jun-2022
Abstract
Preeclampsia (PE) still causes maternal-fetal-naonatal morbidity and mortality. We attempted to find correlation of Ductus Venosus Doppler velocimetry, pulsitility index (MCA/UA ratio) and amniotic fluid index in prediction of perinatal outcome in severe PE. Umbilical artery Pulsatility Index (PI), the presence of absent or reversed end diastolic blood flow, middle cerebral artery PI, and ductus venosus (DV) PI were examined. We found a correlation between an increased DVPI and adverse perinatal outcome, with a sensitivity of 91.7% and a specificity of 76.6%. Also, decreasing amniotic fluid index predicted poor outcomes. Ductus venosus Doppler waveform and decreased amniotic fluid index may be a predictor of poor perinatal outcome in PE.
Keywords
Severe preeclampsia; Adverse outcomes; Fetal doppler indices; Ductus venosus
Introduction
Hypertensive disorders complicating pregnancy are common and form one of the deadly triad along with haemorrhage and infection that results in much of the maternal morbidity and mortality related to pregnancy [1].
Preeclampsia is conventionally considered to be a maternal disorder in which the fetus is an incidental participant. A more complete perception is that the placental problem causes both maternal and foetal syndromes [2].
In most cases, antenatal prediction and identification of the foetuses at risk for preventable morbidity and mortality can help improve the outcome for the mother and her baby.
The past decade has seen an increasing interest in Doppler ultrasound as it provides non-invasive access to uteroplacental and foetal circulation and therefore might yield direct information on the pathophysiology of vascular insufficiency [3].
The umbilical artery, which supplies the placenta, was the first foetal vessel to be evaluated by Doppler velocimetry. Umbilical artery flow in the foetus is usually continuous during all phases of the foetal cardiac cycle. The velocity of blood flow increases with each cardiac systole and then gradually decreases as the blood runs into the placenta. Continuous forward flow is most commonly seen in vessels that supply low resistance organs [3]. When placental resistance increases, the relative amount of blood flow in the umbilical artery during diastole decreases and sometimes reverses. The S/D ratio is a ratio between systolic and diastolic velocity that would thus become higher and higher as the diastolic portion of the flow decreases and placental function theoretically becomes more abnormal. This measurement is considered to be a useful adjunct in the management of pregnancies complicated by foetal growth restriction [4].
Cerebral blood flow has a high degree of autoregulation. This means that cerebral blood flow can vasodilate in the presence of decreased perfusion or vasoconstrict in response to an increase in perfusion pressure. Thus, cerebral blood flow is maintained relatively constant over a large range of blood pressure levels [5].
During hypoxia, blood flow to the brain increases two to threefold in the fetus, while pulmonary, renal, splenic, and gut blood flow decreases. Doppler studies in IUGR demonstrated evidence of redistribution of blood flow in the arterial system with an increase in impedance to flow in the aorta and a decrease in impedance in cerebral circulation. This is evidenced by an increase in the end-diastolic velocity of the Doppler waveform in the cerebral arteries of foetuses and neonates [6].
The compensatory decrease of middle cerebral artery resistance in IUGR may be lost when there is severe hypoxia, metabolic disturbances, or the development of brain edema. (There is a direct correlation between fetal hypoxemia as well as pH and the degree of reduction in impedance to cerebral flow in IUGR) [7].
In most cases, antenatal prediction and identification of the foetuses at risk for preventable morbidity and mortality can help improve the outcome for the mother and her baby [7].
The project's goal
The aim of this work was to find a correlation between Ductus Venosus Doppler velocimetry, pulsitility index, (MCA/UA ratio), and amniotic fluid index in the prediction of perinatal outcome in severe preeclampsia.
Patients and Procedures
This study was a cross-sectional study and included 50 females with a singleton pregnancy suffering from severe preeclampsia and 50 normal females with a singleton pregnancy. Their gestational age ranged between 34 weeks and 38 weeks.
Inclusion criteria
• Age range: 18–40 years.
• Gestational age >34 weeks.
• Singleton pregnancy
• 160 mmHg systolic blood pressure
• 110 mm Hg diastolic blood pressure
• According to the American College of Obstetricians and Gynecologists, gestational proteinuric hypertension can be diagnosed antenatally.
• No obstetric or medical complications of pregnancy apart from preeclampsia.
Exclusion criteria
• Multiple pregnancies
• Preterm pregnancies
• Fetal congenital malformations
• Antepartum still birth.
• Pregnant females with other medical disorders (Diabetes Mellitus, cardiac, liver or renal disease, vaginal bleeding, preterm premature rupture of membranes (PPROM)).
In addition, patients were recruited according to the results of arterial (umbilical and middle cerebral arteries) and venous (ductus venosus) Doppler velocimetry.
Methods
All women were subjected to the following:
• Informed verbal and written consent.
• History and clinical examination
• Routine laboratory investigations
• Ultrasound examinations include:
1. Fetal viability
2. Fetal weight and growth.
3. Intrauterine Growth Restriction (IUGR) was defined as an estimated foetal weight less than the 10th percentile or poor foetal growth on serial ultrasound.
4. Exclusion of foetal anomalies
5. Amniotic fluid index
6. Placental site
7. Foetal biophysical profile
• A Non-Stress Test (NST) was performed on all cases.
• The Doppler study of the feto-placental circulation included the measurement of the umbilical
1. Artery pulse rate (PI). The presence of absent or reversed end diastolic blood flow was recorded.
2. PI of the middle cerebral artery.
3. Ductus Venosus Pulsatility Index for Veins (PIV). The presence of an absent or reversed wave was recorded.
All women were observed in the hospital and were managed by the attending physician.
Immediate pregnancy outcomes obtained were:
1. Gestational age at delivery.
2. Onset of labor: spontaneous or induced.
3. Mode of delivery.
4. Newborn:
a. Apgar score at 1 and 5 minutes.
b. Birth weight.
c. Admission to the Neonatal Intensive Care Unit (NICU).
d. Cord's blood pH.
Administrative Design
Patients are counselled for different diagnostic and treatment options and a written consent is signed. The study was approved by the ethical committee of the hospital.
Statistical Analysis
Using Microsoft Excel software, data collected throughout the history, basic clinical examination, laboratory investigations, and outcome measures were coded, entered, and analyzed.
Results
There were no statistically significant differences in demographic data between the groups studied (Tab. 1.).
| Demographic Data | Group | Total | X2 | P | |||
|---|---|---|---|---|---|---|---|
| Non Hypertensive Group | Hypertensive Group | ||||||
| Gravidity | Multigravida | N | 46 | 46 | 92 | 0 | 1 |
| % | 92.00% | 92.00% | 92.00% | ||||
| PG | N | 4 | 4 | 8 | |||
| % | 8.00% | 8.00% | 8.00% | ||||
| Parity | 0 | N | 7 | 4 | 11 | 4.23 | 0.121 |
| % | 14.00% | 8.00% | 11.00% | ||||
| 01-02 | N | 42 | 46 | 88 | |||
| % | 84.00% | 92.00% | 88.00% | ||||
| >2 | N | 1 | 0 | 1 | |||
| % | 2.00% | 0.00% | 1.00% | ||||
| Abortion | .no | N | 25 | 31 | 56 | 3.21 | 0.22 |
| % | 50.00% | 62.00% | 56.00% | ||||
| Yes | N | 25 | 19 | 44 | |||
| % | 50.00% | 38.00% | 44.00% | ||||
| Total | N | 50 | 50 | 100 | - | - | |
| % | 100.00% | 100.00% | 100.00% | - | - | ||
| - | - | Hypertensive Group | Non Hypertensive Group | - | t | P | |
| - | - | (N=50) | (N=50) | - | |||
| Age | - | - | 29.04 ± 5.12 | 28.82 ± 4.89 | - | 0.219 | 0.827 |
| GA first examination | - | - | 33.76 ± 3.04 | 35.62 ± 1.67 | - | -3.787 | 0.00** |
| GA delivery | - | - | 35.56 ± .2.71 | 37.4 ± 1.44 | - | -4.236 | 0.00** |
| - | - | Hypertensive Group | Non Hypertensive Group | - | t | P | |
| SBP | - | - | 157.0±7.21 | 119.5±6.32 | - | 27.632 | 0.00** |
| DBP | - | - | 102.6±8.82 | 73.5±5.82 | - | 19.467 | 0.00** |
Tab. 1. Obstetric history, Age, SBP and DBP distribution among studied groups distribution between groups.
The hypertensive group had significantly higher IUGR rates and other negative neonatal outcomes (Tab. 2. and 3.)
| Group | Total | X2 | P | ||||
|---|---|---|---|---|---|---|---|
| Non Hypertensive Group | Hypertensive Group | ||||||
| IUGR | No | N | 50 | 38 | 88 | 13.63 | 0.00** |
| % | 100.0% | 76.0% | 88.0% | ||||
| Yes | N | 0 | 12 | 12 | |||
| % | 0.0% | 24.0% | 12.0% | ||||
| Total | N | 50 | 50 | 100 | - | - | |
| % | 100.0% | 100.0% | 100.0% | - | - | ||
Tab. 2. Intra uterine growth retardation distribution between groups.
| Parameters | Group | Total | X2 | P | |||
|---|---|---|---|---|---|---|---|
| Non Hypertensive Group | Hypertensive Group | ||||||
| Preterm | No | N | 43 | 25 | 68 | 14.89 | 0.00** |
| % | 86.0% | 50.0% | 68.0% | ||||
| Yes | N | 7 | 25 | 32 | |||
| % | 14.0% | 50.0% | 32.0% | ||||
| NICU | No | N | 46 | 31 | 77 | 12.7 | 0.00** |
| % | 92.0% | 62.0% | 77.0% | ||||
| Yes | N | 4 | 19 | 23 | |||
| % | 8.0% | 38.0% | 23.0% | ||||
| Death | No | N | 50 | 48 | 98 | 2.04 | 0.15 |
| % | 100.0% | 96.0% | 98.0% | ||||
| Yes | N | 0 | 2 | 2 | |||
| % | 0.0% | 4.0% | 2.0% | ||||
| Total | N | 50 | 50 | 100 | - | - | |
| % | 100.0% | 100.0% | 100.0% | - | - | ||
Tab. 3. Other fetal outcome parameters.
The hypertensive group had a significantly lower mean cord blood pH than the normotensive group (7.290.07 vs. 7.340.05, respectively; p 0.001) (Tab. 4.).
| Parameters | Hypertensive Group | Non Hypertensive Group | t | P |
|---|---|---|---|---|
| DVPI | 1.14 ± 24 | 0.93 ± 0.16 | 5.161 | 0.00** |
| UARI | 1.08 ± 0.22 | 0.91 ± 0.1 | 4.932 | 0.00** |
| MCARI | 0.63 ± 0.11 | 0.62 ± 0.04 | 0.260 | 0.795 |
| Cord blood PH | 7.29 ± 0.07 | 7.34 ± 0.05 | -3.994 | 0.00** |
| AFI | 8.32 ± 3.8 | 16.88 ± 2.43 | -5.985 | 0.00** |
Tab. 4. Comparison between case and control regard Doppler, cord blood PH and AFI.
The pulsatility index of ductus venosus in the group of foetuses of hypertensive mothers with REDF of the umbilical artery group ranged from 0.9 to 2.64 (mean =1.28 0.5). The pulsatility index of ductus venosus in the AEDF of the umbilical artery group ranged from 0.59 to 0.83 (mean=0.73 0.09). The pulsatility index of ductus venosus in the normal flow of the umbilical artery group ranged from 0.45 to 0.88 (mean=0.59 0.09). In table 5, the mean pulsatility index was significantly higher in the REDF group than in the AEDF group and normal flow (P = 0.001) (Tab. 5.)
| Group (REDF) (n = 10) |
Group (AEDF) (n = 20) |
Group (normal flow) (n = 30) |
F | P | |
|---|---|---|---|---|---|
| DV PI Mean ± SD Range |
* 1.28 ± 0.5 0.9-2.64 |
* 0.73 ± 0.09 0.59-0.83 |
0.59 ± 0.09 0.45-0.88 |
37.2 | 0.001* |
| Reversed A wave(cm/s) Mean ± SD Range |
* 56.8 ± 2.5 53-62 |
47.2 ± 3.0 42-51 |
45.2 ± 4.7 38-59 |
32.8 | 0.001* |
| IUGR N (%) |
9(75%) | 3(25%) | 0(0%) | 30.8 | 0.001* |
Tab. 5. Ductus venosus PI in hypertensive group in relation to umbilical artery dopplerand IUGR.
The APGAR score was significantly lower in the hypertensive group than in the normotensive group (5.221.7 vs. 7.120.89 at one minute and 7.222.25 vs. 8.980.86 at five minutes, respectively) (Tab. 6.).
| Hypertensive Group | Non Hypertensive Group | t | P | |
|---|---|---|---|---|
| APGAR at one minute | 5.22 ± 1.7 | 7.12 ± 0.89 | -6.203 | 0.00** |
| APGAR at five minute | 7.22 ± 2.25 | 8.98 ± 0.86 | -5.155 | 0.00** |
| Baby Weight | 2026.56 ± 655.5 | 2936.0 ± 435.0 | -8.174 | 0.00** |
Tab. 6. APGAR and baby weight distribution between group.
A significantly lower mean cord blood pH in the group of abnormal ductus venosus flow than the group with abnormal arterial flow only and the group of normal arterial and venous flow (7.17 ± 0.05, 7.20 ± 0.05 and 7.23 ± 0.06 respectively; p < 0.001). NICU admission was significantly higher in the group of abnormal ductus flow than the group of abnormal arterial flow only and the group of normal arterial and venous flow (100%, 72.5% and 45% respectively; p < 0.001), the mean birth weight in the group of abnormal ductus venosus flow was significantly lower than those of the group of abnormal arterial flow only and the group of normal arterial and venous flow (1377 ± 42 gm, 1878 ± 63 gm and 2177 ± 619 gm respectively; p < 0.001) (Tab. 7.).
| Parameters | Group abnormal ductus venosus flow (n = 10) |
Group abnormal arterial flow (n = 20) |
Group normal arterial and venous flow (n = 30) |
P |
|---|---|---|---|---|
| Cord blood PH | 7.17 ± 0.05 | 7.20 ± 0.05 | 7.23 ± 0.06 | 0.001* |
| NICU admission (%) | 100% | 72.5% | 45% | 0.001* |
| Mean birth weight (gm) | 1377 ± 42 | 1878 ± 63 | 2177 ± 619 | 0.001* |
| - | IUGR N(12) | No IUGR N(38) | T | P |
| Reversed A wave N(%) |
10(20 %) | 0(0%) | 5.161 | 0.045 |
Tab. 7. Cord blood PH, NICU admission and mean birth weight in hypertensive group in relation to abnormal umbilical artery and Ductus venosus Doppler.
DVPI sig negatively correlated with cord blood pH, AFI, APGARat one minute and APGAR at five minute, AFI positively with cord blood pH, APGARat one minute and APGAR at five minute and weight.) (Tab. 8.). The DV had a sensitivity of 91.7 % and a specificity of 76.6 %, it appears to have potential for use as a method of fetal assessment for preeclamptic pregnancy, Sensitivity and Specificity for pH regard IUGR was 83.3% and 86.8% respectively while Sensitivity and Specificity for AFI regard IUGR was 75.0% and 100.0% respectively (Tab. 9. and 10.) (Fig. 1. and 2.).
| Parameters | DVPI | AFI | |
|---|---|---|---|
| Cord blood PH | R | -.614-** | .530** |
| P | .000 | .000 | |
| AFI | R | -.586-** | 1 |
| P | .000 | ||
| APGAR at one minute | R | -.284-* | .759** |
| P | .046 | .000 | |
| APGAR at five minute | R | -.286-* | .686** |
| P | .044 | .000 | |
| Weight | R | -.224- | .403** |
| P | .119 | .004 | |
Tab. 8. Correlation of DVPI, Cord blood PH, AFI, APGARat one and five minutes and fetal weight.
| Test Result Variable(s) | Area | Cutoff | P | 95% Confidence Interval | Kappa agreement | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| DVPI | 0.853 | >1.2 | 0.00** | 0.748 | 0.958 | 0.69 | 91.7% | 76.6% |
Tab. 9. Sensitivity and Specificityfor DVPI cut off regard IUGR.
| Test Result Variable(s) | Area | Cut off | P | 95% Confidence Interval | Kappa agreement | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Cord blood PH | 0.883 | <7.22 | 0.00** | 0.774 | 0.991 | 0.74 | 83.3% | 86.8% |
| AFI | 0.954 | <7.6 | 0.00** | 0.945 | 0.999 | 0.76 | 75.0% | 100.0% |
Tab. 10. Sensitivity and Specificityfor PH and AFI cutoffs regard IUGR.
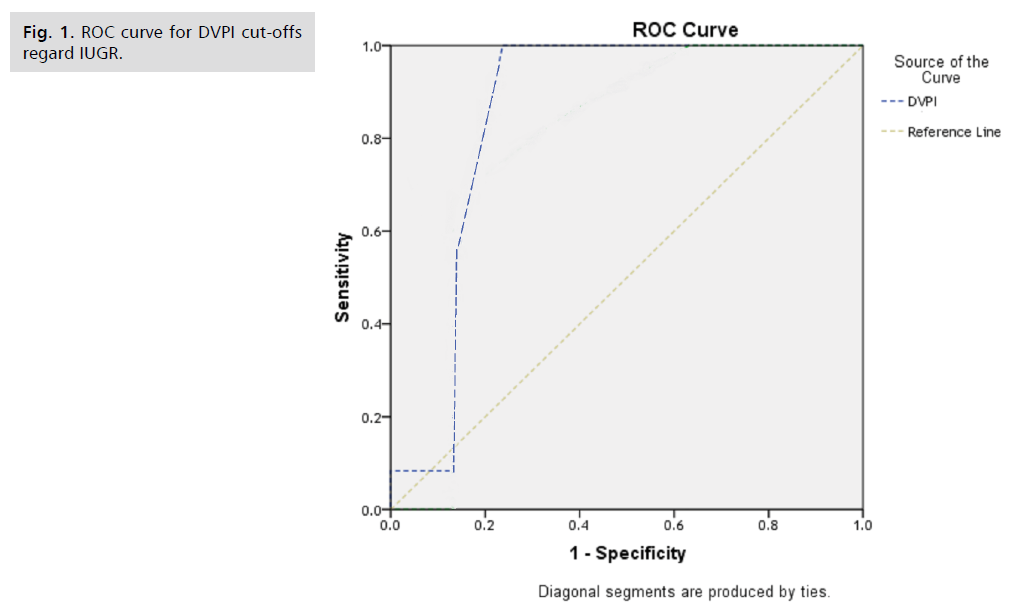
Fig 1. ROC curve for DVPI cut-offs regard IUGR.
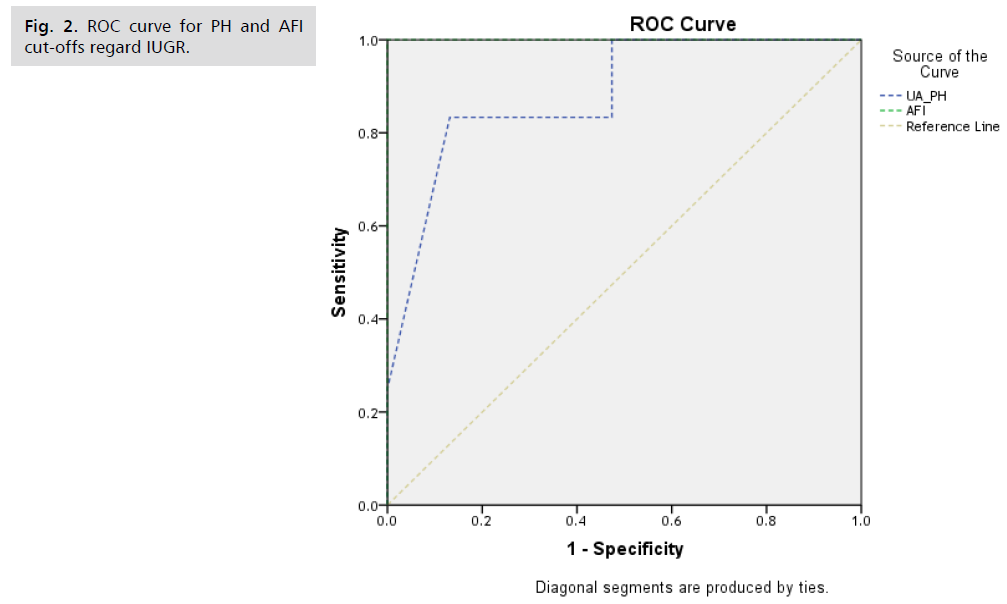
Fig 2.ROC curve for PH and AFI cut-offs regard IUGR.
Discussion
Severe placental insufficiency is related to early and extreme growth restriction. Serial Doppler measurements of the umbilical artery, middle cerebral artery, and ductus venosus are commonly used for monitoring compromised pregnancies. The majority of the severely compromised foetuses have pathological venous velocimetry, most notably an increased pulsatility in the ductus venosus as a sign of impaired myocardial function [4].
Hofstaetter et al. studied the effect of venous Doppler velocimetry in the surveillance of severely compromised foetuses. In their prospective study, 154 growth-restricted foetuses were recruited according to Lauren’s umbilical artery blood velocity classification into 4 groups: group of normal umbilical artery blood velocity, group of increased umbilical artery PI but still forward flow, group of absent flow in end diastole in the umbilical artery, and lastly, a group of reversed flow in the umbilical artery. Venous blood flow analysis in these 4 groups reported a significant increase in mean ductus venous PIV in the group of reversed flow than in the group of absent flow, group of forward flow with high PI in the umbilical artery and group of normal umbilical artery blood flow (1.66 0.31, 0.66 0.27, and 0.66 0.39, respectively; p 0.01). This was in agreement with our study as the mean ductus venosus PIV was significantly higher in the group of foetuses of hypertensive mothers with REDF of the umbilical artery (1.28 0.5). The pulsatility index of ductus venosus in the AEDF of the umbilical artery group was 0.73 0.09. The pulsatility index of ductus venosus in the normal flow of the umbilical artery group range was (0.59 0.09). The mean pulsatility index was significantly higher in the REDF group than in the AEDF group and normal flow (P = 0.001) [8].
Alves et al. evaluated the relationship between ductus venosus Doppler findings on the day of delivery and postnatal outcomes in pregnancies with absent or reversed end-diastolic (ARED) flow in the umbilical arteries. Their retrospective study involved 103 new borns from pregnancies with a diagnosis of ARED flow on Doppler velocimetry of the umbilical artery. Cases were divided into two groups according to the flow during atrial contraction (a-wave) in the ductus venosus on the day of delivery: 20 cases with absent or reversed flow in the ductus venosus and 83 cases with continuous positive flow. Analysis of blood flow in the umbilical arteries on the day of delivery showed a larger number of cases with reversed end diastolic flow (15/20, 75%) in the group of absent or reversed flow in ductus compared to the group of positive continuous flow (27/83, 32.5%) (p = 0.001). This goes with our study where the number of reversed waves was significantly higher in groups with AREDF in the umbilical artery than those without (p 0.001) [9].
Baschat et al. reported a lower birth weight in cases of abnormal ductus venosus flow. This was in agreement with our study where the frequency of IUGR was highest in cases with abnormal venous flow. In addition, the mean birth weight in the group of abnormal ductus venosus flow was significantly lower than those of the group of abnormal arterial flow only and the group of normal arterial and venous flow (1377 42 gm, 1878 63 gm, and 2177 619 gm, respectively; p 0.001) [10].
The cut-off value found in Arvalho et al.'s study for predicting acidemia at birth using ductus venosus PIV was similar to that found in Hofstaetter et al.'s study for predicting perinatal mortality: PIV 0.76 vs. 0.99 (area under the curve 0.818 vs. 0.84) [9].
Similarly, we reported a significantly lower mean cord blood pH in the group of abnormal ductus venosus flow than in the group with abnormal arterial flow only and the group of normal arterial and venous flow (7.17 0.05, 7.20 0.05, and 7.23 0.06, respectively; p 0.001).
Similarly, we reported a significantly lower mean cord blood pH in the group of abnormal ductus venosus flow than in the group with abnormal arterial flow only and the group of normal arterial and venous flow (7.17 0.05, 7.20 0.05, and 7.23 0.06, respectively; p 0.001).
In our study, the frequency of NICU admission was significantly higher in the group of abnormal ductus flow than in the group of abnormal arterial flow only and the group of normal arterial and venous flow (100%, 72.5%, and 45%, respectively; p 0.001). In contrast, Alves et al. found no significant difference between the group of absent or reversed flow in ductus venosus and the group of continuous positive regarding the frequency of NICU admission; however, there was a significantly higher frequency of intraventricular haemorrhage (p = 0.02) and of neonatal hypoglycemia (p = 0.01) in the group of absent or reversed flow in ductus venosus. This was in agreement with Muller et al., who observed a higher frequency of intracranial haemorrhage (50%) among cases with absent or reversed ductus venosus flow than cases with positive flow (13%) [9].
In the study of Mari et al., they found that in pregnancies not complicated by preeclampsia, Doppler changes are predictable for foetal outcome and if an abrupt cause does not intervene, i.e. placenta abruption, they occur in a sequential manner day after day until the foetus develops cardiac failure and dies if it is not delivered. However, in pre-eclamptic patients, these sequential changes either do not occur or occur in a short time. For example, in IUGR, a foetus with forward Umbilical Artery (UA) diastole and normal Ductus Venosus (DV) Doppler may develop UA reversed flow and DV reversed flow in a few hours if the mother develops preeclampsia [11].
According to Hecher et al., the ductus venosus does not change before non-stress tests all the time, but the majority of the authors describe the ductus venosus Doppler velocimetry as indicating changes before modifications in other biophysical tests as reported by Ferrazzi et al., Romero et al., and Baschat. In the study of Barbosa et al., the ductus venosus seems to be one of the best vessels to monitor in compromised fetuses, helping to decide when to deliver [12]
Bilardo and Baschat reported that the likelihood of stillbirth increases with the degree of venous Doppler abnormality. Venous Doppler findings that are particularly ominous are the absence, or reversal of the ductus venous a-wave and biphasic/triphasic umbilical venous pulsations. In the setting of a 25% stillbirth rate in a preterm severe IUGR population, these Doppler findings had a 65% predictive sensitivity and 95% specificity [13].
In our study, the frequency of absent or reversed end diastolic flow in the umbilical artery was higher in those with IUGR than in those without IUGR (p 0.001), and also the reversed wave in the ductus venosus was significantly higher in those with IUGR than those without IUGR (p = 0.045).
Farine et al. reported that AREDF in the umbilical artery is commonly associated with severe IUGR, oligohydramnios, and adverse perinatal outcome. Mandruzzato et al. found that there is a significant difference in perinatal mortality for absent end diastolic flow (20%) vs. reversed end diastolic flow (68%) in the umbilical artery [14].
In the present study, the frequency of IUGR was significantly higher in the group of abnormal ductus venosus and abnormal arterial flow of the umblical artery compared with that in the group of normal arterial and venous flow (p 0.01). The rate of admission to the NICU was also significantly higher in those with IUGR than those without (100% vs. 59.3%; p 0.001).
Gerber et al. considered immediate delivery to be an indication for cases with IUGR and associated with an absent or reverse end-diastolic blood flow in the umbilical artery, especially for the mean gestational age of 31 weeks; in these circumstances, expectant management did not improve long-term foetal prognosis. Although there is still a major problem of prematurity. However, the decision in the IUGR foetus with AREDF in the umbilical artery They recommended that a larger randomised study with a statistically significant sample size focus on the subgroup of 24-30 weeks of pregnancy [14].
Conclusion
The finding here are re confirmation of one the preceding knowledge, however, we believe that area/institute –specific data is mandatory for the practice.
References
- Cunningham FG. Hypertensive disorders in pregnancy. Williams Obstet. 2005.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911-919.
- James DK, Steer PJ, Weiner CP, et al. High risk pregnancy e-book: management options-expert consult. Elsevier Health sci. 2010.
- Arias F, Bhide AG, Arulkumaran S, et al. Practical Guide to High Risk Pregnancy and Delivery-E-Book. Elsevier Health sci. 2008.
- Stevens W, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237-248.
- Sandlin AT, Chauhan SP, Magann EF. Clinical relevance of sonographically estimated amniotic fluid volume: polyhydramnios. J Ultrasound Med. 2013;32(5):851-863.
- von Dadelszen P, Payne B, Li J, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. The Lancet. 2011;377(9761):219-227.
- Homer CS, Brown MA, Mangos G, et al. Non-proteinuric pre-eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens. 2008;26(2):295-302.
- Agrawal S, Agrawal V, Yadav S. Comparative study of amniotic fluid index in normal & high risk pregnancy complicated by PIH. Ind J Obst Gynaecol Res. 2015;2:242-245.
- Baschat AA, Cosmi E, Bilardo CM, et al. Predictors of neonatal outcome in early-onset placental dysfunction. Obstet Gynecol. 2007;109(2 Part 1):253-61.
- Miller J, Turan S, Baschat AA. Fetal growth restriction. Semin Perinatol. 2008;32:274-280.
- Hecher K, Bilardo CM, Stigter RH, et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol. 2001;18:564-570.
- Bilardo CM, Wolf H, Stigter RH, et al. Relationship between monitoring parameters and perinatal outcome in severe, early intrauterine growth restriction. Ultrasound Obstet Gynecol. 2004;23:119-125.
- Ferrazzi E, Bozzo M, Rigano S, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth‐restricted fetus. Ultrasound Obstet Gynecol. 2002;19:140-146.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Mohamed I Taema*, Nada Alayed#, Salwa Neyazi, Ibrahim Ali and Amr Sobhy1Department of Obstetrics and Gynecology, King Saud University, Saudi Arabia
2Department of Obstetrics and Gynecology, King Saud University, Saudi Arabia
3Department of Obstetrics and Gynecology, King Saud University, Saudi Arabia
#Equally contribution
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.