Research - (2024) Volume 19, Issue 3
Control nausea and vomiting during chemotherapy in pregnant women with non-small cell lung cancer
Manar Hameed Mohammed1*, Noor H. Naser2, Sammar Jassim Mahan3 and Ashwaq Najemaldeen Abbas4Received: 02-Sep-2024, Manuscript No. gpmp-24-148582; Editor assigned: 03-Sep-2024, Pre QC No. P-148582; Reviewed: 14-Sep-2024, QC No. Q-148582; Revised: 21-Sep-2024, Manuscript No. R-148582; Published: 30-Sep-2024
Abstract
Background: Chemotherapy-Induced Nausea and Vomiting (CINV) seriously affect cancer patients both physically and emotionally, therefore compromising their quality of life and maybe discouraging more treatment. The several kinds of CINV need for varied treatment schedules, usually involving corticosteroids, 5-HT3 receptor antagonist, and NK1 receptor antagonist.
Methods: 150 pregnant women with non-small cell Lung Cancer were divided into Five groups: 1st group administer Ondansetron (OND); 2nd group administered Dexamethasone (Dex); 3rd group administered Metoclopramide (Met); 4th group on combination OND plus Dex and 5th group on Aprepitant plus Dexamethasone (Apr+Dex). the main objective was to assess the percentage of patients experiencing total CINV within each group.
Results: Of the 150 women, 71 (44%) received MEC and 90 (56%) received HEC. The 4th group showed 60% Complete Control, 13% Complete Protection, and 27% Complete Response which consider better results and remarkable degree of protection as compared with other groups. 1st and 2nd groups showed non-significant difference (p>0.05).
Conclusion: The results of the study led to guidelines for the use of serotonin and corticosteroids to prevent CINV in patients with NSCLC undergoing chemotherapy.
Keywords
Chemotherapy; Pregnant women; Lung cancer
Introduction
The medulla oblongata's Vomiting Center (VC) is responsible for coordinating the vomiting reaction. This is achieved by the VC combining a spectrum of peripheral and central impulses, which are known as the peripheral and central pathways, respectively [1]. Via the peripheral pathway, abdominal vagal afferents transmit stimuli including pharyngeal stimulation and stomach/duodenal distension [2]. Abdominal vagal afferent fibers express many receptors including 5-HT3, Neurokinin (NK) 1, and cholecystokinin-1, which can induce the emetic reaction. The main arbiter of this reaction is 5-HT3 [3]. The symptoms of Chemotherapy-Induced Nausea and Vomiting (CINV) may manifest at various stages of the treatment regimen. Immediate CINV, which is characterized by vomiting within 24 hours of the initial administration of chemotherapy, is primarily mediated by 5-HT3 receptors [4] Delayed Chemotherapy-Induced Nausea and Vomiting (CINV) occurs between 24 and 5 hours following chemotherapy and is largely mediated by substance P binding to NK1 receptors in the central nervous system [5].
Aim
The purpose of the study is to examine the reliability and effectiveness of antiemetic medicines in the treatment of NSCLC during pregnancy.
Method
The prospective study enrolling 150 pregnant women with NSCLC stage 2 from different government hospitals. The women were divided into five groups depend on type of antiemetic medication; 1st group administration ONS 8 mg IV for acute and 8 mg for orally for delay phase; 2nd group administration Dex 8 mg IV and 0.5 mg orally; 3rd group Met 10mg IV and 10 mg orally; 4th group administration combination ONS plus Dex 8mg plus 8 mg for IV and 8 mg plus 0.5mg for oral; and 5th group administration aprepitant plus Dex130 mg IV plus 0.5 mg and 125 mg orally plus 0.5 mg.
Exclusion criteria was history of alcohol consumption, their history of motion sickness or pregnancy-related vomiting
Acute NV was defined by a Likert score of at least 1 for nausea or vomiting on day 1 (chemotherapy) and delayed NV by any day between days 1 and 7 after chemotherapy. Investigators used the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) to evaluate vomiting events [6].
Statistical analysis
This study used descriptive analysis. The sample's demographics, clinical features, incidence and severity of delayed emesis, number of emetic episodes, and time till emesis were summarized using descriptive statistics. The chi-squared test was used to compare delayed nausea and vomiting in men and women. Multivariate logistic regression was also performed to examine how additional clinical and demographic factors affect delayed emesis rates.
Results
Patients' demographics properties
This study enrolled 150 people between October 2023 and January 2024. Included in the study were 100 patients (66.67%) who received HEC and 50 (33.33%) who received MEC (Tab. 1.).
| Characteristics | % (n) | P Value |
|---|---|---|
| Age (years) | ||
| 20 to 40 years | 28 (42) | 0.033 |
| 41 to 60 years | 72 (108) | |
| Marital status | ||
| Single | 20 (30) | 0.022 |
| Married | 80 (120) | |
Tab. 1. Demographic specifications of the patients undergoing CT (n=150).
Antiemetic regimens
The rate of MEC induced acute emesis on day 1 as reported by the patients on their diary card can be seen in Tab. 2. and Fig. 1. Ondansetron plus corticosteroid combination was administered to 95.5% of patients during acute emesis, indicating a high level of protection against this phase. This rate is similar to that of patients treated with Aprepitant plus corticosteroid combination (87.2%). When it came to controlling acute emesis, there was no significant difference (p>0.05) between the combinations of aprepitant with corticosteroid, dexamethasone alone (83.7%), and ondansetron alone (83.4%). Nonetheless, there was insignificant difference (p>0.05) between the acute and delay phases in the management of acute emesis.
| Category | OND n=41 | Dex. n=30 | Met. n=30 | OND + Dex n=30 | Apr.+ Dex. n=30 | P value |
|---|---|---|---|---|---|---|
| Acute (<24 hr) (day 1) | 83.4 | 85.7 | 53.6 | 95.5* | 87.2 | 0.002 |
| Delayed (>24 hr) (days 2–7) | 75.3 | 77.1 | 50.1 | 91.2* | 82.4 | 0.005 |
Tab. 2. Percentage of acute and delayed emesis by chemotherapeutic agents.
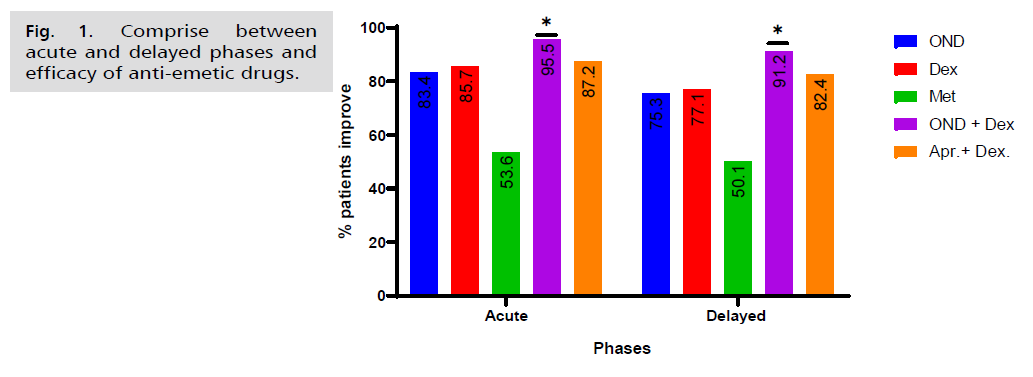
Fig. 1. Comprise between acute and delayed phases and efficacy of anti-emetic drugs.
The endpoints of the all-patient groups, complete response, complete protection, and complete control were examined. The findings showed that, for delayed (days 2-7) antiemetic medication, patients that took single medication as Dexamethasone our results showed that there is insignificantly difference (p>0.05) between it and Ondansetron while we found higher proportion of both medications when compared with Metoclopramide to achieve delayed complete control (40% and 37% vs. 20%, P=0.003). prior to adjusting for measured confounders statistically, patients taking Ondansetron or Dexamethasone alone were approximately 1.3-1.8 times more likely to exhibit delayed complete control than patients taking Metoclopramide (unadjusted odds ratio [OR]=1.25, 1.83 respectively. 95% confidence interval [CI]=-31.61 to 31.61).
On another hand, we noticed that the combination of Ondansetron with Dexamethasone exhibits higher percent of complete control when compared with Aprepitant plus dexamethasone combination (60% vs. 43%) (Unadjusted odds ratio [OR]=1.4. 95% confidence interval [CI]=-11.21 to 21.23) (Tab. 3. and Fig. 2.).
| Protocol | Administration | Complete Control |
Complete Protection | Complete Response |
|---|---|---|---|---|
| Ondansetron (A) | MEC n=41 | 15 (37%) | 9 (22%) | 17 (41%) |
| Dexamethasone (B) | MEC n=30 | 12 (40%) | 7 (23%) | 11 (37%) |
| Metoclopramide (C) | MEC n=30 | 6 (20%) | 11 (37%) | 13 (43%) |
| Ondansetron + Dexamethasone (D) | MEC n=30 | 18 (60%) | 4 (13%) | 8 (27%) |
| Aprepitant + dexamethasone (E) | MEC n=30 | 13 (43%) | 8 (27%) | 9 (30%) |
Tab. 3. CINV control.
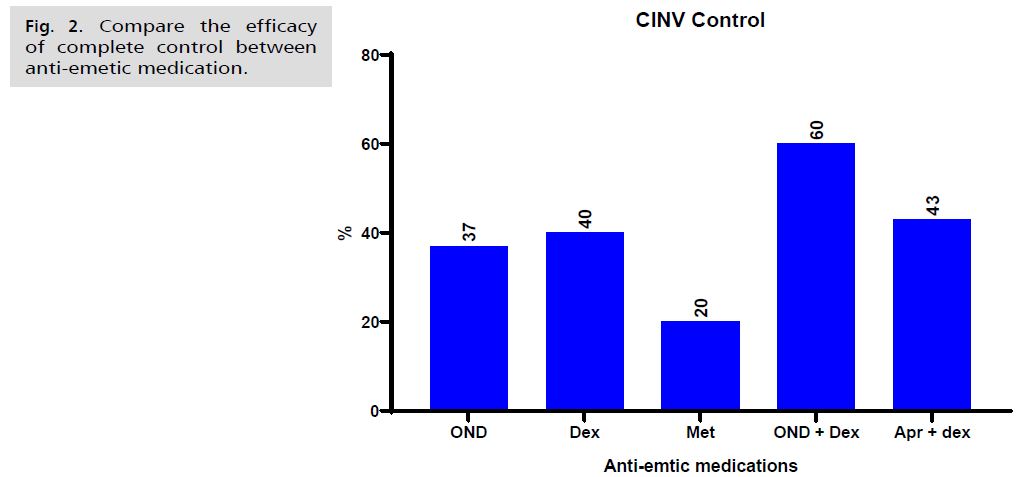
Fig. 2. Compare the efficacy of complete control between anti-emetic medication.
Discussion
Since CINV is a serious adverse reaction to chemotherapy, accurately estimating the likelihood of its occurrence is crucial. Our research consistently showed that accurately predicting whether a patient will experience CINV after receiving HEC or MEC is challenging. As a result, we recommend following current guidelines and not reducing antiemetics. However, despite confirming previously reported risk factors for CINV, our study did not identify sufficient predictors. We consistently found that younger age was a risk factor for acute nausea, acute vomiting, and delayed nausea, aligning with findings from previous research.
The study's findings indicate that in the acute phase, approximately half of the patients who received HEC reported CINV, whereas in the delayed phase, more than half of them experienced nausea. According to the Likert scale for the severity of CINV, we observed that the score was high on the first day and gradually declined until the seventh day, while the score was zero during the delay phase and gradually increased until the seventh day, when the maximum score was recorded.
There is still a need for effective treatment of nausea during both the immediate and prolonged periods in patients who receive High Emetogenic Chemotherapy (HEC) and Moderately Emetogenic Chemotherapy (MEC) [7]. Even though use the single drug such as NK-1 receptor antagonist alone or a 5-HT 3 receptor antagonist or combination, dexamethasone has demonstrated advantages in patients undergoing high emetogenic chemotherapy with cisplatin or cyclophosphamide-doxorubicin [8,9].
We found that combination of serotonin receptor antagonists in combination with DEX had highly efficacy in reduce CINV as compared with other anti-emetics while other studies investigated controversial, Herrington, et al. conducted a comparison trial with aprepitant, palonosetron, and DEX alone, There were found that no significant differences in emesis or nausea which aligns with our study findings [10]. Kang, et al. found that oral aprepitant, combined with ondansetron, with or without DEX, effectively prevents chemotherapy-induced nausea and vomiting in patients undergoing chemotherapy, compared to controls or those treated only with ondansetron, with or without DEX [11].
Conclusion
The combination of Ondansetron with corticosteroids is considered a standard treatment regimen for preventing CINV in patients with NSCLC undergoing chemotherapy.
Recommendation
We recommended that healthcare providers should using Ondansetron with corticosteroids as part of their treatment guideline for chemotherapy induces CINV to minimize the risk.
Authors' Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- He Y, Zheng J, Ye B, et al. Chemotherapy-induced gastrointestinal toxicity: Pathogenesis and current management. Biochem Pharmacol. 2023:115787.
- Browning KN, Carson KE. Central neurocircuits regulating food intake in response to gut inputs—preclinical evidence. Nutrients. 2021;13(3):908.
- Mahendra IN, Setiawan WA. Current Management of CINV. Eur J Med Health Sci. 2023;5(3):55-59.
- Rahman AA, Masango P, Stavely R, et al. Oxaliplatin-induced damage to the gastric innervation: role in nausea and vomiting. Biomol. 2023;13(2):276.
- Aapro M. CINV: Still troubling patients after all these years. Support Care Cancer. 2018;26:5-9.
- Vazin A, Eslami D, Sahebi E. Evaluating the antiemetic administration consistency to prevent chemotherapy-induced nausea and vomiting with the standard guidelines: A prospective observational study. Ther Clin Risk Manag. 2017:1151-1157.
- Ng TL, Hutton B, Clemons M. Chemotherapy-induced nausea and vomiting: Time for more emphasis on nausea?. Oncologist. 2015;20(6):576-583.
- Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer. 2016;24:675-682.
- Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of Chemotherapy-Induced Nausea and Vomiting (CINV): A systematic review and meta-analysis. Support Care Cancer. 2016;24:2381-2392.
- Herrington JD, Jaskiewicz AD, Song J. Randomized, placebo‐controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy‐induced nausea and vomiting. Cancer. 2008;112(9):2080-2087.
- Kang HJ, Loftus S, Taylor A, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting in children: A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(4):385-394.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Manar Hameed Mohammed1*, Noor H. Naser2, Sammar Jassim Mahan3 and Ashwaq Najemaldeen Abbas42Department of Pharmaceutical Chemistry, College of Pharmacy, Al-Zahraa University for Women, Karbala, Iraq
3Department of Pharmacy, Al-Zahrawi University College, Karbala, Iraq
4Department of Pharmacy, College of Dentistry, University of Sulaymaniyah, Sulaymaniyah Governorate, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.