Research - (2022) Volume 17, Issue 3
Comparison between agonist trigger with HCG luteal phase supplementation vs. HCG trigger with progesterone luteal phase supplementation in antagonist controlled hyperstimulation cycle regarding clinical pregnancy rate
Maii Nawara*, Alaa El Din El Guindy, Walid Hitler, Sherif Yehia and Laila Aly FaridReceived: 17-Jul-2022, Manuscript No. gpmp-22-69452; Editor assigned: 18-Jul-2022, Pre QC No. P-69452; Reviewed: 28-Jul-2022, QC No. Q-69452; Revised: 10-Aug-2022, Manuscript No. R-69452; Published: 30-Sep-2022
Abstract
Objective: We compared agonist trigger and HCG luteal support vs. standard HCG trigger and progesterone luteal supplementation in antagonist controlled hyperstimulation cycle as regards to clinical pregnancy rate.
Patients and Methods: The study was conducted on 100 women undergoing IVF treatment. They were randomized through a computer-generated list into two groups. Group I (n=50): Standard protocol HCG trigger with progesterone luteal support, and Group II (n=50): New protocol agonist trigger with HCG luteal support.
Results: Group II, compared with Group I, showed non-significant higher pregnancy rate. Group II showed much better compliance from patients: this was considered owing to the progesterone injection being administered intramuscularly or subcutaneously.
Conclusion: Low dose HCG luteal phase support with agonist trigger in antagonist cycles provided similar or higher (non-significant) pregnancy rates, compared with conventional HCG trigger and progesterone luteal phase support. This protocol provided better patient satisfaction and compliance.
Keywords
Abortion Agonist trigger; HCG luteal phase supplementation; HCG trigger; Progesterone luteal phase supplementation; Antagonists; Clinical pregnancy rate
Introduction
Aetiology of luteal phase defect in stimulated IVF cycles has been debated for more than two decades. Initially, it was thought that the removal of large quantities of granulosa cells during the oocyte retrieval (OR) might diminish the most important source of progesterone synthesis by the corpora lutea, leading to a defect of the luteal phase. However, this hypothesis was disproved when it was established that the aspiration of a preovulatory oocyte in a natural cycle neither diminished the luteal phase steroid secretion nor shortened the luteal phase [1,2]. Since it was found that the corpus luteum can be rescued by the administration of hCG, this treatment has become the standard care for luteal support since the late 1980s [3].
By stimulating the corpora lutea, hCG is an indirect form of luteal support. It is known to generate an increase in E2 and progesterone concentrations, thus rescuing the failing corpora lutea in stimulated IVF cycles [4,5].
A large bolus of hCG has been routinely used for final follicular maturation and has for many years been considered the gold standard for cycles of IVF. However, because it was associated with excessive risk of ovarian hyperstimulation syndrome (OHSS) in high responders, an alternative trigger agent was needed to safely induce oocyte maturation in such patients. The GnRH agonist (GnRHa) trigger was not effective in ovarian stimulation protocols that used daily GnRHa for pituitary down-regulation, and therefore the practical use of GnRHa trigger awaited the availability and wider use of GnRH antagonists [6].
The first randomized controlled studies using the GnRH-a trigger concept had to be prematurely discontinued owing to unacceptably high early pregnancy loss rates, caused by a severe Corpus luteum dysfunction, which could not be solved by standard luteal phase support (LPS) policies. Efforts resulted in the development of two concepts: the modified LPS, which uses a small bolus of 1.500 IU hCG administered on the day of oocyte retrieval, in combination with a standard LPS to overcome the luteal phase insufficiency and the intensive LPS, using supplementation with exogenous steroids (progesterone and estradiol) [7-9].
The administration of 125 IU hCG daily resulted in significantly higher progesterone levels during the mid-luteal phase as compared with the standard protocol in which vaginal micronized progesterone was administered on a daily basis. Moreover, the daily low-dose luteal hCG circumvented the sharp incline in the progesterone serum level and the supra-physiological steroid level traditionally seen during the early luteal phase after hCG trigger. Thus, use of the GnRHa trigger plus low-dose hCG for luteal phase support appeared to resemble more the relatively slow increase in progesterone concentration observed during the natural cycle in the early luteal phase than the hCG trigger. It is notable that the mean levels of hCG at no point exceeded the normal physiological LH level [10].
The aim of this study was to compared agonist trigger and HCG luteal support vs. standard HCG trigger and progesterone luteal supplementation in antagonist controlled hyperstimulation cycle as regards to clinical pregnancy rate.
Patients and Methods
Study design
Prospective Interventional randomized pilot study on patients undergoing controlled ovarian hyperstimulation.
Study setting
All patients were recruited from a private infertility clinic.
Study population
Study group: Women attending to the fertility clinic for IVF cycles
Inclusion criteria
• Age between 20 and 39 years
• Body mass index between 18 and 30
• Unexplained infertility or male factor infertility
Exclusion criteria
• Any Endocrinological disorder
• Hyperprolactenemia
• PCO
• Hypo or hyper thyrodism
• More than 2 previous attempts of IVF
• Any uterine anatomical anomaly.
Consent
Informed written consent was taken from all participants before recruitment in the study, and after explaining the purpose and procedures of the study.
Randomization
The study was conducted on 100 women undergoing IVF treatment. They will be randomized at the outpatient clinic by an employee on the basis of a computer generated list into two groups.
• Group I (n=50): Standard protocol HCG trigger with progesterone luteal support
• Group II (n=50): New protocol Agonist trigger with HCG luteal support
Allocation concealment
Dark sealed envelopes containing the intervention derived from computer generated list were created by a third party not involved in the allocation process then randomization was performed by picking one envelope for each patient from sequenced number envelopes by a nurse not involved in the study.
Ethical considerations
The study was approved from the ethics committee of the Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University.
All women included were subjected to the following:
• History taking with particular emphasis on past medical history, menstrual history and infertility workup.
• General, abdominal and local examination.
• BMI will be assessed.
• Venous blood samples for the assessment of CBC, FSH, LH, Prolactin, E2 used by the clinic as a part of their protocol.
• Transvaginal (TV) ultrasound (U/S) on day 3 of non-stimulated cycles will be done by transvaginal probe of 5-9 MHZ. Any patient discovered to have uterine or tubal pathology will be excluded.
• All patients received a fixed dose of 150-300 IU recombinant FSH (Gonal-F; Sereno Laboratories, Madrid, Spain) for ovarian stimulation according to age, BMI and antral follicle count (AFC).
• After 6 days of stimulation, FSH will be adjusted according to ovarian response.
• Premature LH surge was prevented with 0.25 mg of a GnRH antagonist (Cetrotide; Serono International, Geneva, Switzerland) starting on day 6 when two or more follicles reach a size of 18–20 mm, trigger of ovulation was done and followed by luteal phase support according to the protocol assigned for each group.
Group 1
A single dose of 0.2 mg triptorelin (Decapeptyl® Ipsen Pharmaceutical Company, France) and follow up with daily 125 IU HCG injections.
Group 2
A single dose of HCG 10000 IU was given followed by progesterone supplementation with 100mg IM (Prontogest®).
Ovum pick up
36 hours after HCG injection, the transducer was connected to the ultrasound system. The direction of the guide beam was checked. The puncturing needle was be connected to an aspiration apparatus attached by a fixation ring to the front and rear ends of the vaginal transducer, thereby defining the direction of puncture corresponding to the guide beam on the ultrasound image.
The aspiration was checked using test tubes. The uterus, both ovaries and iliac vessels will be identified by the visualization in both planes. The distance between the upper pole of the vagina and the ovary was closely evaluated (care was taken to avoid intestinal or vascular interposition).
Depth localization of the closest accessible follicle (distance from the upper vaginal pole to the center of the follicle) will be done. Needle was pushed forcefully to the center of the follicle (Aspiration pressure 90-100mmHg).
IVF- ICSI
Intracytoplasmic sperm injection will be performed on metaphase II oocytes using the direct penetration technique, fertilization results will be assessed 16 to 19 hours after ICSI. Fertilization will be considered normal by the presence of two pronuclei. Oocyte degeneration will be identified by collapse of cytoplasmic contents and separation from the zona. Failed fertilization will be defined by the absence of the pronuclei.
Embryo transfer
Embryo transfer will be done on day 3 to 5 using cook catheter under ultrasound guide at a distance about 1-1.5 cm from the fundus by the same gynecologist.
Defining pregnancy
Biochemical pregnancy was determined by positive pregnancy test performed 10 days after embryo transfer. Clinical pregnancy will be defined by the presence of gestational sac using transvaginal ultrasound performed 4 weeks after embryo transfer.
Elimination of bias
• All patients underwent ovulation induction for IVF using antagonist protocol
• All ovum pickups were done by the same surgeon with the same probe, setting and ultrasound machine
• Laboratory samples were analyzed in the same laboratory.
• Oocyte study were assessed by the same embryologist
• Transfer of 2 Embryos was done
• Luteal phase support was supplemented through both protocols.
Preparation of HCG injection
• HCG was found to be stable for up to 60 days after constitution, 5000 units of HCG was diluted on 20 ml of Distilled Water, then the patient was asked to further divide that among 2 10 ml syringes.
• To achieve the 125 IU a day only 0.5 ml was needed subcutaneously to maintain luteal phase support.
Data management and analysis
The collected data was revised, coded, tabulated and introduced to a PC using Statistical package for Social Science (SPSS 15.0.1 for windows; SPSS Inc, Chicago, IL, 2001). Data was presented and suitable analysis was done according to the type of data obtained for each parameter.
Descriptive statistics:
1. Shapiro Wilk test was used to evaluate normal distribution of continuous data Mean, Standard deviation ( ± SD) and range was used for parametric numerical data, while Median and Interquartile range (IQR) for non-parametric numerical data.
2. Frequency and percentage of non-numerical data.
Analytical statistics:
1. Student T Test was used to assess the statistical significance of the difference between two study group means.
2. Mann Whitney Test (U test) was used to assess the statistical significance of the difference of a non-parametric variable between two study groups.
3. Chi-Square test was used to examine the relationship between two qualitative variables
4. Correlation analysis (using spearman's method): To assess the strength of association between two quantitative variables. The correlation coefficient denoted symbolically "r" defines the strength and direction of the linear relationship between two variables.
P- value: Level of significance:
• P>0.05: Non significant (NS)
• P< 0.05: Significant (S)
• P<0.01: Highly significant (HS)
Sample size justification: No sufficient data is available to generate a specific hypothesis. An estimated number of 50 patients in each group could be recruited. No sample size has been calculated
Results
Among group 1 cases (Tab. 1.), the mean age was 29.22 ± 4.09. The mean AMH was 3.18 ± 1.6 and the mean total dose of HMG was 3170.5 ± 852.2. Fig. 1. shows the flow chart of study cases. Among group 1 cases (Tab. 2.), the mean total number of oocyte, number of MII, retrieved embryos and transferred embryos was 14.6 ± 6.32, 11.64 ± 6.17, 9.06 ± 5.3, and 2 ± 0.0 respectively. The mean number of total days of stimulation was 10.8 ± 1.4. The pregnancy rate among group 1 cases was 52% (Tab. 3.). Among group 2 cases, the mean age was 29.82 ± 3.22. The mean AMH was 3.068 ± 2.2 and the mean total dose of HMG was 3052.0 ± 505.02 (Tab. 4.).
| Variables | Mean | ± SD | Minimum | Maximum |
|---|---|---|---|---|
| Age | 29.22 | 4.09 | 19.00 | 38.00 |
| AMH | 3.18 | 1.66 | .20 | 8.50 |
| Total dose of HMG | 3170.50 | 852.21 | 2025.00 | 4950.00 |
Tab. 1. Description of personal and clinical data among group 1 cases (control group).
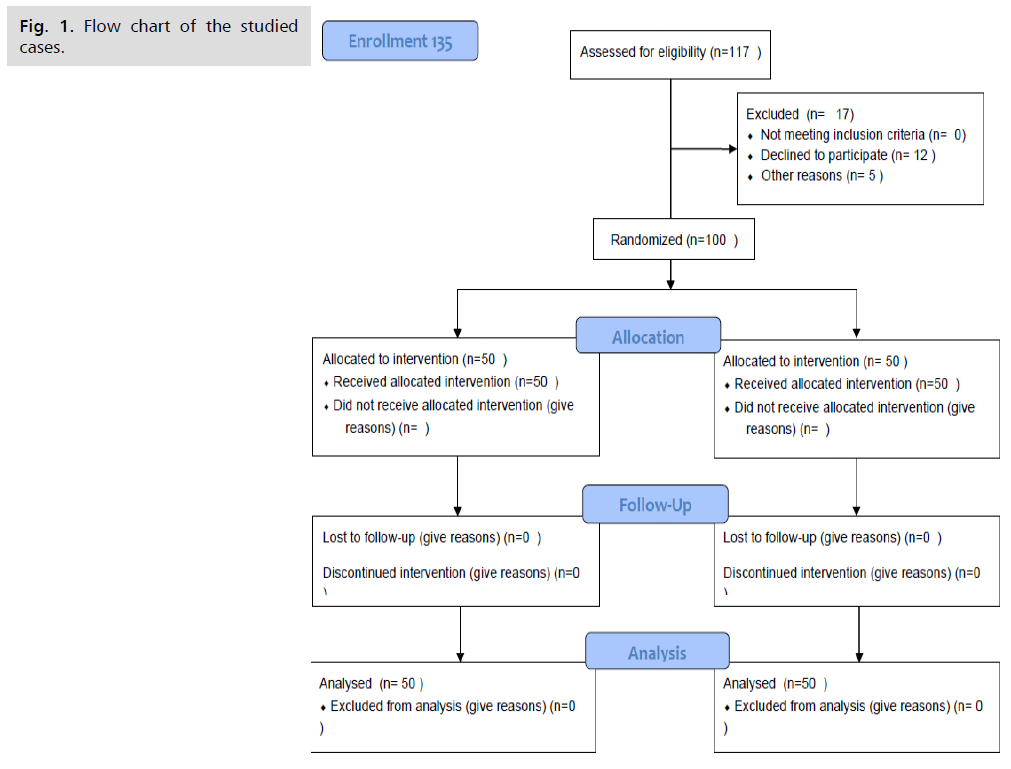
Fig 1. Flow chart of the studied cases.
| Variables | Mean | ± SD | Minimum | Maximum | Median | IQR* | |
|---|---|---|---|---|---|---|---|
| Total number of Oocyte | 14.64 | 6.32 | 4.00 | 26.00 | 14.0 | 9.0 | 19.0 |
| Total number of MII | 11.64 | 6.17 | 2.00 | 25.00 | 11.0 | 6.0 | 16.0 |
| Total number of Embryos | 9.06 | 5.35 | 2.00 | 22.00 | 8.0 | 4.0 | 13.0 |
| Number of embryos Transferred | 2.00 | .00 | 2.00 | 2.00 | 2.0 | 2.0 | 2.0 |
| Total days of stimulation | 10.82 | 1.41 | 9.00 | 16.00 | 11 | 10 | 11 |
Tab. 2. Description of total number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation among group 1 cases.
| Variables | N | % | |
|---|---|---|---|
| Pregnancy | Negative | 24 | 48.0% |
| Positive | 26 | 52.0% | |
Tab. 3. Description of treatment outcome (Pregnancy) among group 1 cases.
| Variables | Mean | ± SD | Minimum | Maximum |
|---|---|---|---|---|
| Age | 29.82 | 3.22 | 23.00 | 36.00 |
| AMH | 3.06 | 2.20 | 0.70 | 10.00 |
| Total dose of HMG | 3052.00 | 505.02 | 1800.00 | 4125.00 |
Tab. 4. Description of personal and clinical data among group 2 cases (treatment group).
Among group 2 cases, the mean total number of oocyte, number of MII, retrieved embryos and transferred embryos was 9.4 ± 3.03, 6.98 ± 2.5, 6.16 ± 2.64, and 2 ± 0.0 respectively. The mean number of total days of stimulation was 10.56 ± 1.07 (Tab. 5.). The pregnancy rate among group 2 cases was 56% (Tab. 6.). There was no significant difference between both study groups as regard age, AMH and total dose of HMG (Tab. 7.) (Fig. 2.). There was a highly significant difference between both study groups as regard total number of oocyte, MII, and retrieved embryos. However, no significant difference was found as regard Number of embryo Transferred and total days of stimulation (Tab. 8.).
| Variables | Mean | ± SD | Minimum | Maximum | Median | IQR* | |
|---|---|---|---|---|---|---|---|
| Total number of Oocyte | 9.42 | 3.03 | 5.00 | 18.00 | 9 | 7 | 11 |
| Total number of MII | 6.98 | 2.50 | 2.00 | 12.00 | 7 | 6 | 9 |
| Total number of Embryos | 6.16 | 2.64 | 2.00 | 12.00 | 6 | 3 | 8 |
| Number of embryos Transferred | 2.00 | .00 | 2.00 | 2.00 | 2 | 2 | 2 |
| Total days of stimulation | 10.56 | 1.07 | 9.00 | 13.00 | 11 | 10 | 11 |
Tab. 5. Description of total number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation among group 2 cases (treatment group).
| Variables | N | % | |
|---|---|---|---|
| Pregnancy | Negative | 22 | 44.0% |
| Positive | 28 | 56.0% | |
Tab. 6. Description of treatment outcome (Pregnancy) among group 2 cases (treatment group).
| Variables | Group | P | Sig | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Trial | |||||||||||
| Mean | SD | Median | IQR‡ | Mean | SD | Median | IQR‡ | |||||
| Age | 29.22 | 4.09 | 29 | 27 | 32 | 29.82 | 3.22 | 30 | 27 | 33 | 0.41* | NS |
| AMH | 3.18 | 1.66 | 3 | 2 | 4 | 3.06 | 2.20 | 2.5 | 1.2 | 4.3 | 0.76** | NS |
| HMG | 3170.5 | 852.21 | 3000 | 2475 | 3750 | 3052.0 | 505.02 | 3000 | 2700 | 3375 | 0.4* | NS |
Tab. 7. Comparison between Group 1 (control) and group 2 (trial) as regard personal and clinical data
Fig 2. Comparison between group 1 (control) and group 2 (trial) as regard to Age and AMH.
| Variables | Group | P | Sig | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Trial | |||||||||||
| Mean | SD | Median | IQR‡ | Mean | SD | Median | IQR‡ | |||||
| Oocyte | 14.64 | 6.23 | 14 | 9 | 19 | 9.42 | 3.03 | 9 | 7 | 11 | 0.001* | HS |
| MII | 11.56 | 6.20 | 11 | 6 | 16 | 6.98 | 2.50 | 7 | 6 | 9 | 0.001* | HS |
| Embryos | 9.04 | 5.40 | 9 | 4 | 13 | 6.16 | 2.64 | 6 | 3 | 8 | 0.001* | HS |
| Number of embryo Transferred | 2.00 | .00 | 2 | 2 | 2 | 2.00 | .00 | 2 | 2 | 2 | 1.0** | NS |
| Days of stimulation | 10.82 | 1.41 | 11 | 10 | 11 | 10.56 | 1.07 | 11 | 10 | 11 | 0.26* | NS |
Tab. 8. Comparison between Group 1 (control) and group 2 (trial) as regard number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation.
There was no significant difference between both studies groups as regard pregnancy rate, as 52% of group 1 cases were pregnant compared to 56% of group 2 cases (Tab. 9.). Among all cases, there was a highly significant positive correlation between AMH and each of number of oocyte (Fig. 3.), MII and total number of retrieve embryos (Tab. 10.) (Fig. 4.). There was no significant difference between pregnant and non-pregnant group 1 cases as regard age, AMH and total dose of HMG (Tab. 11.).
| Variables | Group | P | Sig | ||||
|---|---|---|---|---|---|---|---|
| Control | Trial | ||||||
| N | % | N | % | ||||
| Pregnancy | Negative | 24 | 48.0% | 22 | 44.0% | 0.68* | NS |
| Positive | 26 | 52.0% | 28 | 56.0% | |||
Tab. 9. Comparison between Group 1 (control) and group 2 (trial) as regard pregnancy rate.
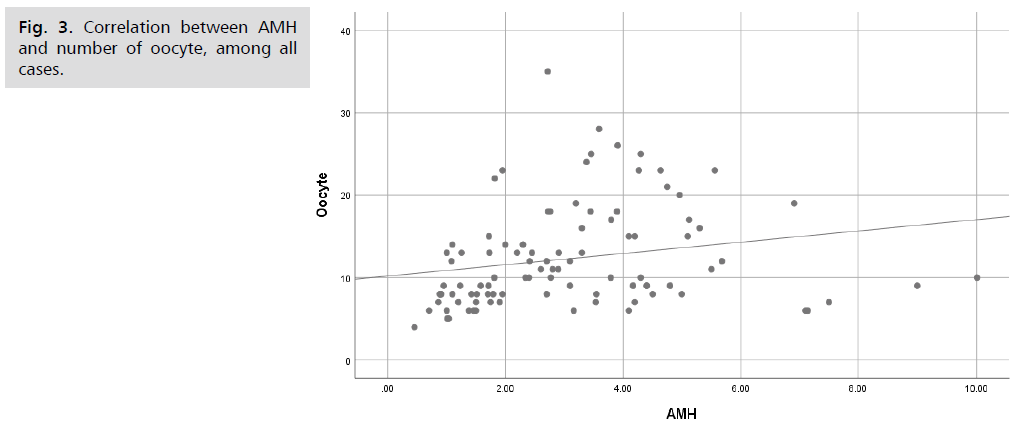
Fig 3. Correlation between AMH and number of oocyte, among all cases.
| Variables | Oocyte | MII | Total Embryos | |
|---|---|---|---|---|
| AMH | R* | 0.363 | 0.322 | 0.250* |
| P | 0.0001 | 0.0001 | 0.013 | |
| sig | HS | HS | S | |
Tab. 10. Correlation between AMH and number of oocyte, MII and total embryos among all cases.
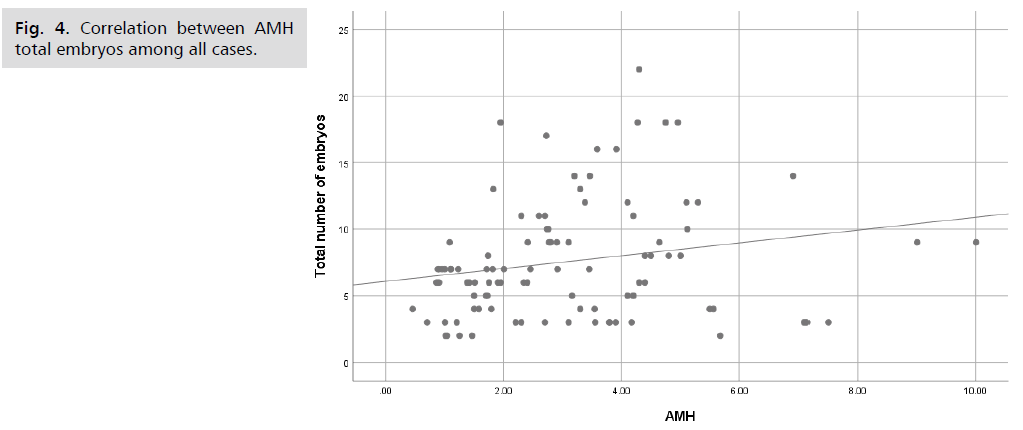
Fig 4. Correlation between AMH total embryos among all cases.
| Variables | Pregnancy | P | Sig | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||||||
| Mean | SD | Median | IQR‡ | Mean | SD | Median | IQR‡ | |||||
| Age | 30.04 | 4.15 | 31 | 28 | 32 | 28.46 | 3.95 | 29 | 26 | 31 | 0.192* | NS |
| AMH | 2.83 | 1.64 | 2 | 2 | 4 | 3.50 | 1.65 | 3 | 3 | 4 | 0.168* | NS |
| HMG | 3393.75 | 793.49 | 3300 | 2813 | 3975 | 2964.42 | 867.29 | 2719 | 2250 | 3300 | 0.102* | NS |
Tab. 11. Comparison between pregnant and non-pregnant group 1 cases (controls) as regard personal and clinical data.
There was no significant difference between pregnant and non-pregnant group 1 cases as regard total number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation (Tab. 12.). There was no significant difference between pregnant and non-pregnant group 2 cases as regard age, AMH and total dose of HMG (Tab. 13.). There was no significant difference between pregnant and non-pregnant group 1 cases as regard total number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation (Tab. 14.).
| Variables | Pregnancy | P | Sig | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||||||
| Mean | SD | Median | IQR‡ | Mean | SD | Median | IQR‡ | |||||
| Oocyte | 14.04 | 6.82 | 13 | 9 | 18 | 17.27 | 8.48 | 18 | 12 | 23 | 0.192 | NS |
| MII | 10.00 | 5.66 | 10 | 5 | 14 | 13.00 | 6.44 | 14 | 7 | 18 | 0.062 | NS |
| Total number of embryos | 7.83 | 4.87 | 7 | 4 | 11 | 10.15 | 5.72 | 10 | 6 | 14 | 0.109 | NS |
| Number of embryos Transferred | 2.0 | 0 | 2 | 2 | 2 | 2.0 | 0 | 2 | 2 | 2 | 1.0** | NS |
| Days of stimulation | 10.87 | 1.33 | 11 | 10 | 12 | 10.77 | 1.50 | 11 | 10 | 11 | 0.909 | NS |
Tab. 12. Comparison between pregnant and non-pregnant group 1 cases (controls) as regard total number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation.
| Variables | Pregnancy | P | Sig | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||||||
| Mean | SD | Median | IQR‡ | Mean | SD | Median | IQR‡ | |||||
| Age | 30.41 | 3.05 | 31 | 27 | 33 | 29.36 | 3.34 | 29 | 27 | 31 | 0.256 | NS |
| AMH | 2.71 | 1.40 | 3 | 1 | 4 | 5.75 | 13.81 | 2 | 1 | 5 | 0.289 | NS |
| HMG | 3034.09 | 568.23 | 3000 | 2550 | 3300 | 3066.07 | 459.63 | 3225 | 2875 | 3375 | 0.827 | NS |
Tab. 13. Comparison between pregnant and non-pregnant group 2 cases (treatment) as regard personal and clinical data.
| Variables | Pregnancy | P | Sig | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||||||
| Mean | SD | Median | IQR‡ | Mean | SD | Median | IQR‡ | |||||
| Oocyte | 9.86 | 3.26 | 9 | 8 | 11 | 9.07 | 2.85 | 9 | 7 | 10 | 0.373* | NS |
| MII | 7.41 | 2.13 | 8 | 6 | 9 | 6.64 | 2.75 | 6 | 6 | 9 | 0.287* | NS |
| Embryos | 6.73 | 2.35 | 7 | 5 | 8 | 5.71 | 2.81 | 6 | 3 | 7 | 0.181* | NS |
| Number Transferred | 2.00 | .00 | 2 | 2 | 2 | 2.00 | .00 | 2 | 2 | 2 | 1.0** | NS |
| Days Of stimulation | 10.73 | .88 | 11 | 10 | 11 | 10.43 | 1.20 | 11 | 9 | 11 | 0.33* | NS |
Tab. 14. Comparison between pregnant and non-pregnant group 2 cases (treatment) as regard total number of oocyte, MII, retrieved embryos, transferred embryos and days of stimulation.
Discussion
Corpus Luteum does not need supraphysiologic levels of LH/hCG to secrete high amounts of P. During the natural menstrual cycle, the LH level in the luteal phase seldom exceeds
5-10 IU/L and is still capable of eliciting P levels most commonly in excess of 25-35 nmol/L. When ten CLs are present, each will secrete P in amounts similar to the natural menstrual cycle when exposed to physiologic concentrations of LH/hCG. Collectively, this results in high concentration of P. In both arms of our trial each arm was 50 patients with a total of 100 patients for the trial. The aim was to see if the traditional trigger in antagonist protocols and using standard luteal phase support was the same as the proposed regiment which was the introduction of the agonist trigger with microdoses of HCG for luteal phase support.
The benefit was that using agonist trigger was less likely to result in hyperstimulation in high yielding cycles as well as a good oocyte maturation and embryo quality [11].
In this study the mean age of patients was 29 in both groups, AMH was 3.1 in group 1 vs. 3.06 in group 2, The total dose administered was 3170 in group 1 vs. 3052 in group 2 which is non-significant between both groups (Tab. 10.).
The total number of oocytes was slightly different between both groups in terms of yield of 14.6 in group 1 vs. 9.42 in group 2, this could be attributed to a few patients with a higher total oocyte yield. The number MII oocytes was 11.5 vs. 6.98 (Tab. 11.).
The same observation was found by Humaidan P, et al. [12] who found that in a prospective randomized controlled study although significantly more oocytes were retrieved Following 10,000IU HCG than following buserelin at 0.5 mg dose for final oocyte n trigger. Apparently there was no difference in the maturation and MII percentage in both protocols however there is a difference between HCG and Agonist in final maturation. HCG has a greater effect on cAMP and steroidogenic action than does LH, whereas LH has a greater effect on extracellular signal-related kinase and AKT signaling, which are anti-apoptotic proliferative signals. This difference in action is hypothesized to relate to their physiological roles in the normal menstrual cycle and in early pregnancy, GnRHa activates pituitary GnRH receptors to release both endogenous LH and FSH, whereas hCG possesses only LH-like activity Whereas the mid-cycle FSH surge is not critical for oocyte maturation to occur, FSH is known to increase LH receptor expression in granulosa cells and additionally may directly play a role in the expansion of cumulus oocyte complexes and oocyte maturation [13].
Andersen CY, et al. [10] did not find any difference in the number of oocyte yield with agonist trigger which was identical whether HCG or agonist is used. In this study however there was a difference between both groups which could be attributed to the random selection of patients and the increased yield in group 1 random patients was predetermined irrelative to the trigger itself. This difference did not affect the results in this study as both groups had an equal number of embryos transferred (2 embryos).
More importantly as regards to clinical pregnancy rates, group 1 had a 52% pregnancy rate while group 2 had an even higher pregnancy rate of 56% although non statistically significant it shows agonist trigger with modified Luteal phase support group to have a slightly higher pregnancy rate. This is in agreement with the original trial by Andersen CY, et al., where he found out that the pregnancy rate was 37% in the trial group vs. 40% in the control group. The lower pregnancy rate is probably due to the single embryo transfer which was 1.08 + -0.05 vs. 1.1 + - 0.06. This indicates that the low dose HCG was enough to maintain the luteal phase compared to the standard luteal phase support. It is also believed that the low-dose hCG stimulation of the CL will also stimulate the production of a number of other substances believed to be of importance for early pregnancy, including other sex steroids, peptide hormones, cytokines and growth factors [14].
Another explanation was put forward by Gurbuz AS, et al. [15] who found that the time intervals during early embryo development were shorter in GnRHa-triggered cycles. Previous studies have compared early and late cleaving embryos and found that significantly more early cleaving embryos were good-quality embryos and the transfer of early cleavage embryos resulted in higher implantation and pregnancy rates.
There was a significant negative correlation between total dose of HMG and AMH, this is in accordance with Anckaert E, et al. [16] who found that the higher the starting AMH the lower the starting dose of HMG as well as the total dose of HMG involved, this is also in accordance with
A positive correlation between AMH and total number of oocytes retrieved Table, MII oocytes and total number of Embryos formed. This is in agreement with Vidales, et al., 2017 who stated that Basal AMH serum concentration was the strongest predictor of oocyte yield. This was also the case for Zheng H, et al. [17] who found AMH to positively correlate to number of oocytes retrieved as well a useful tool in terms of counseling patients regarding their risk of cycle cancellation depending on the cutoffs of poor response [17].
There were no cases of hyperstimulation noted within this trial as a result of daily micro dose HCG. HCG helps support the CL longer as well as provides a better quality endometrium but due to the longer half-life increases the release of vasoactive peptides leading to higher rates of OHSS. Agonist triggers causes a more defective corpus luteum which leads to less vasoactive peptides but a poorer quality endometrium which is why low dose daily HCG would provide better endometrial receptivity as well as corpus luteum function and luteal phase support [12].
Our results are in agreements with the studies of Humaidan P, et al., and show that micro dose HCG together with agonist trigger in antagonist protocol can be used safely as luteal support without increase in hyper stimulation syndrome.
During this study the main difficulty was teaching the patients how to adjust the doses of the HCG, in the study performed by Andersen CY, et al. [10] after diluting the HCG to the required dose it was tested and verified to maintain the required concentration. The patients once passing the learning curve there was no dropout rate of treatment or miscompliance. This is supported by Gandel DL, et al. [18] who found that women who had prior experience with SC and IM injections had a 75% preference rate towards SC injections.
Conclusion
During this study we have been able to provide data showing that introducing this method of low dose HCG luteal phase support with agonist trigger in antagonist cycles provided no lesser outcomes in terms of pregnancy rates vs. conventional HCg trigger and progesterone luteal phase support conventionally used. This was also achieved while providing a more physiological response and better patient satisfaction and compliance.
References
- Kerin JF, Broom TJ, Ralph MM, et al. Human luteal phase function following oocyte aspiration from the immediately preovular graafian follicle of spontaneous ovular cycles. BJOG: Int J Obstet Gynaecol. 1981;88(10):1021-1028.
- Tavaniotou A, Devroey P. Effect of human chorionic gonadotropin on luteal luteinizing hormone concentrations in natural cycles. Fertil Steril. 2003;80(3):654-5.
- Whelan III JG, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril. 2000 May 1;73(5):883-96.
- Leth-Moller K, Jagd SH, Humaidan P. The luteal phase after GnRHa trigger-understanding an enigma. Int J Fertil Steril. 2014;8(3):227.
- Hutchinson-Williams KA, DeCherney AH, Lavy G, et al. Luteal rescue in in vitro fertilization-embryo transfer. Fertil Steril. 1990;53(3):495-501.
- Shapiro BS, Andersen CY. Major drawbacks and additional benefits of agonist trigger—not ovarian hyperstimulation syndrome related. Fertil Steril. 2015;103(4):874-8.
- Humaidan P, Polyzos NP. Human chorionic gonadotropin vs. gonadotropin-releasing hormone agonist trigger in assisted reproductive technology—“The king is dead, long live the king!”. Fertil Steril. 2014;102(2):339-41.
- Liang IT, Huang HY, Wu HM, et al. A gonadotropin releasing hormone agonist trigger of ovulation with aggressive luteal phase support for patients at risk of ovarian hyperstimulation syndrome undergoing controlled ovarian hyperstimulation. Taiwan J Obstet Gynecol. 2015;54(5):583-7.
- Blockeel C, De Vos M, Verpoest W, et al. Can 200 IU of hCG replace recombinant FSH in the late follicular phase in a GnRH-antagonist cycle? A pilot study. Hum Reprod. 2009;24(11):2910-6.
- Andersen CY, Elbaek HO, Alsbjerg B, et al. Daily low-dose hCG stimulation during the luteal phase combined with GnRHa triggered IVF cycles without exogenous progesterone: a proof of concept trial. Hum Reprod. 2015;30(10):2387-95.
- Deepika K, Suvarna R, Sumi M, et al. HCG trigger versus GnRH agonist trigger in PCOS patients undergoing IVF cycles: frozen embryo transfer outcomes. JBRA Assist Reprod. 2021;25(1):48.
- Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
- Abbara A, Clarke SA, Dhillo WS. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev. 2018;39(5):593-628.
- Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593-7.
- Gurbuz AS, Gode F, Uzman MS, et al. GnRH agonist triggering affects the kinetics of embryo development: a comparative study. J Ovarian Res. 2016;9(1):1-6.
- Anckaert E, Smitz J, Schiettecatte J, et al. The value of anti-Müllerian hormone measurement in the long GnRH agonist protocol: association with ovarian response and gonadotrophin-dose adjustments. Hum Reprod.. 2012;27(6):1829-39.
- Zheng H, Chen S, Du H, et al. Ovarian response prediction in controlled ovarian stimulation for IVF using anti-Müllerian hormone in Chinese women: A retrospective cohort study. Medicine. 2017;96(13).
- Gandell DL, Bienen EJ, Gudeman J. Mode of injection and treatment adherence: results of a survey characterizing the perspectives of health care providers and US women 18–45 years old. Patient Prefer Adherence. 2019;13:351.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Maii Nawara*, Alaa El Din El Guindy, Walid Hitler, Sherif Yehia and Laila Aly FaridCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.